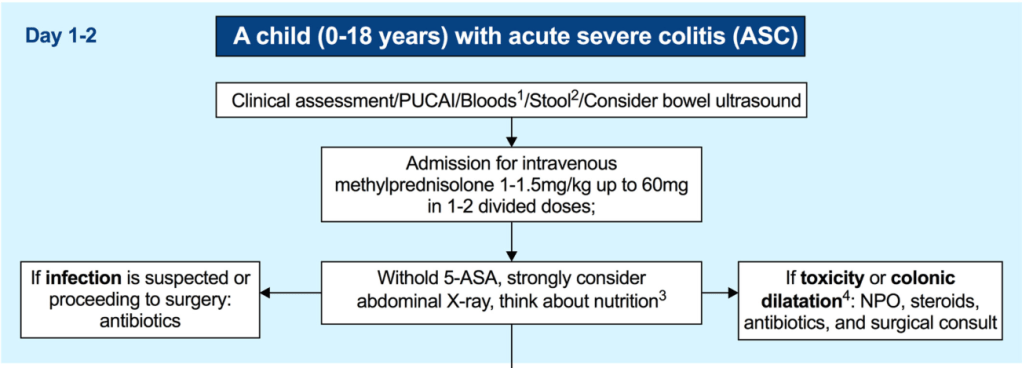
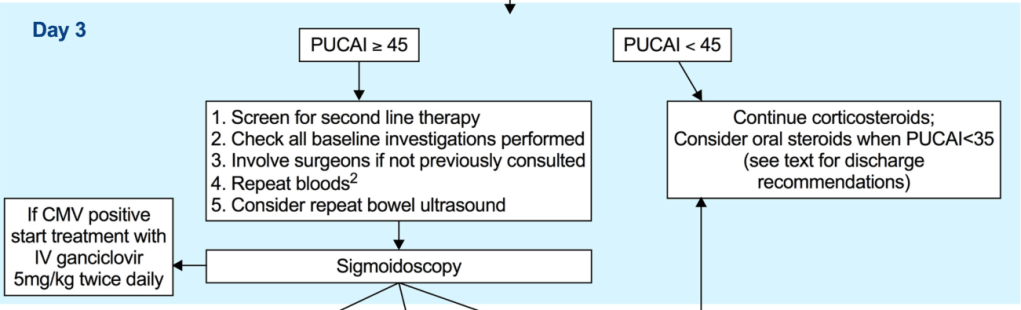
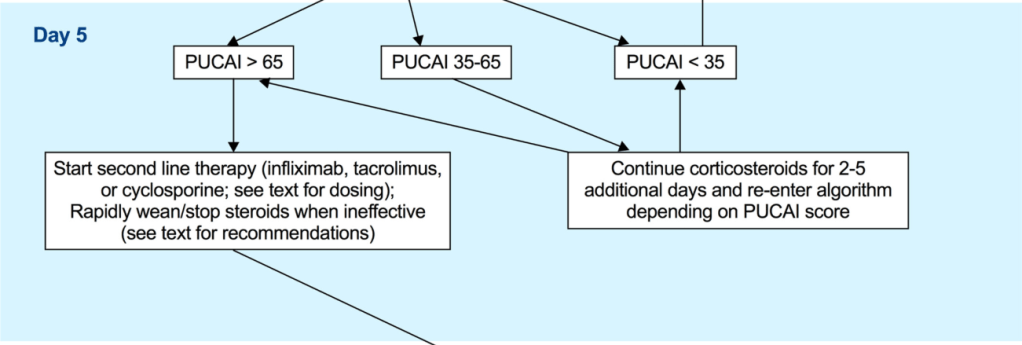
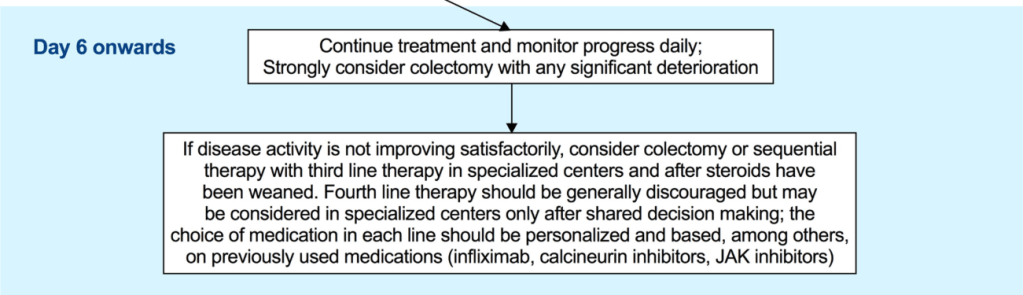
LM Felipez et al. J Pediatr Gastroenterol Nutr. 2025;81:1100–1117. Open Access! North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition position paper on the therapeutic drug monitoring in pediatric inflammatory bowel disease
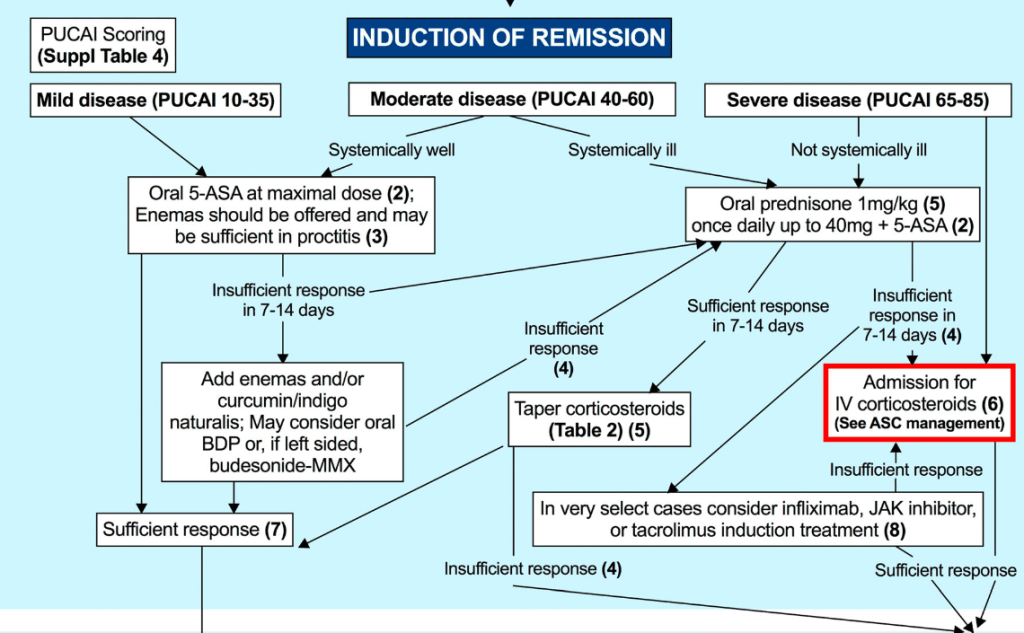
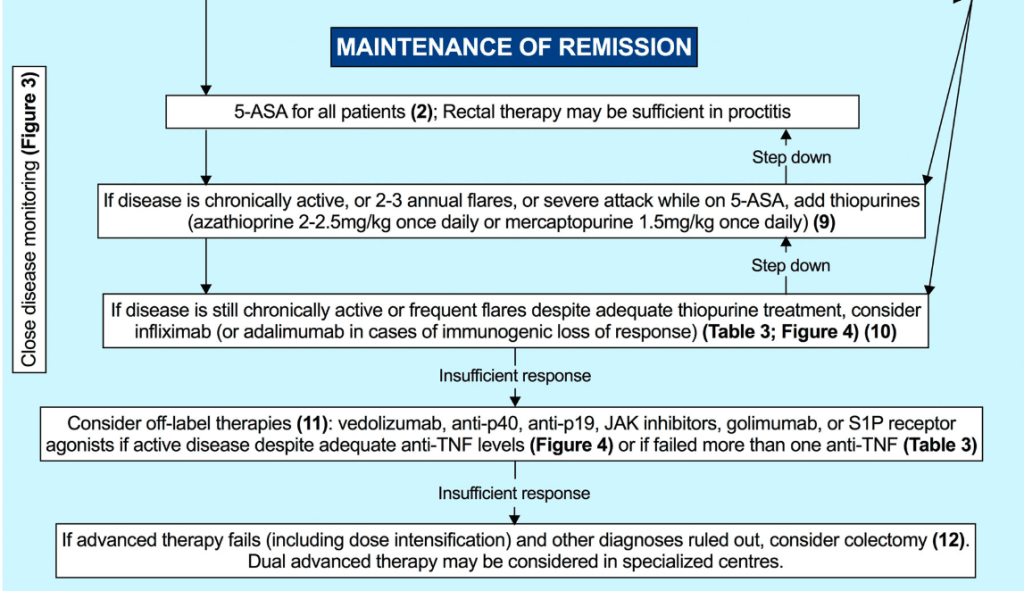
Therapeutic Drug Targets Based on Condition, Medication and Time of Therapy:
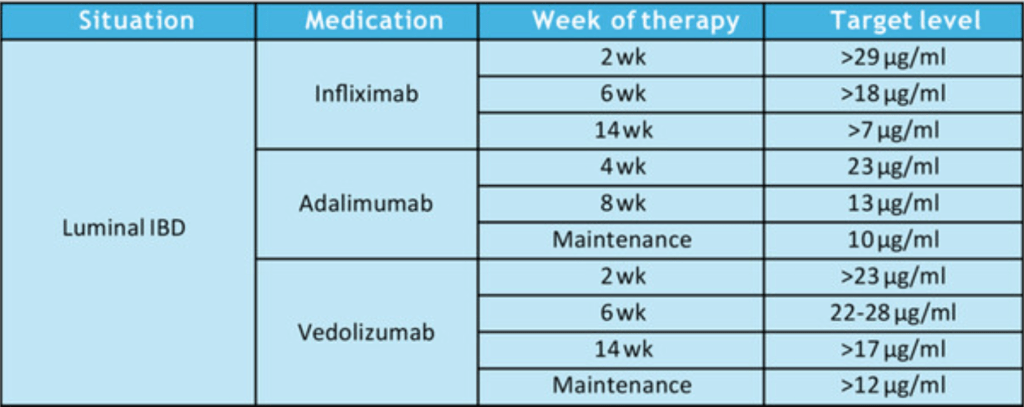
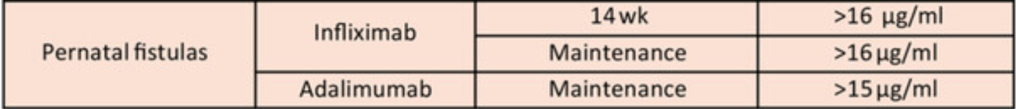

Discussion Points:
- Pediatric Dosing is Different: “Pediatric studies have also determined adult infliximab targets are insufficient…In a prospective pediatric study, Clarkston et al. found that a trough level of 29 μg/mL at 2 weeks is required to achieve both clinical and biologic response. Patients with lower trough levels had 13-fold greater odds of clinical nonresponse. Additionally, a trough of 18 μg/mL at 6 weeks was associated with improved response. Patients with lower trough levels had sixfold greater odds of clinical nonresponse. They also observed that patients who did not achieve a trough >5–7 μg/mL by 14 weeks of therapy had a 21-fold increase in the odds of clinical nonresponse.62“
- Undetectable/very low anti-TNF levels: “If the serum level is extremely low or undetectable, then full re-induction is warranted in addition to dose escalation.”
- Timing of TDM: “As a practice point, TDM is routinely recommended at the end of induction for most patients. We recommend obtaining TDM earlier during induction in at-risk populations, including younger age children, those with hypoalbuminemia, and those with increased inflammatory burden.”
- Maintenance proactive TDM: “Based on prospective randomized trial evidence, we recommend proactive TDM during maintenance every 6–12 months…yearly proactive TDM was associated with 55% reduced risk of developing antidrug antibodies.26“
- Increased Antidrug Antibodies with Lower Infliximab Dosing: “In the pivotal REFINE study on immunogenicity in pediatric IBD, Coleman et al. found that antibodies to infliximab were detected in 68% of patients in the cohort, and starting dose under 7.5 mg/kg was one of the strongest predictors of developing antidrug antibodies.4“
- Higher Doses Prevent Antidrug Antibodies: “The best available evidence for preventing immunogenicity supports initiating therapy with infliximab doses greater than 8 mg/kg, and in the case of hypoalbuminemia, doses greater than 10 mg/kg. For children <40 kg, doses of 200 mg/m2 are more appropriate.”
- Perianal fistulas: “Overall, there is less evidence to support adalimumab use over infliximab for treatment of perianal fistulas. It is possible that adalimumab may have lower efficacy for perianal fistula.105 However, it is unclear if this is inherent to adalimumab, or if it relates to less frequent TDM or less frequent dose escalation in practice.”
- Vedolizumab: “In general, as with other biologic therapies, a higher serum vedolizumab concentration is associated with higher likelihood of treatment response…Multiple studies identified that in patients with IBD (either UC or CD) early trough levels at Week 2132 with a cut off of >23.2 μg/mL or Week 6133, 134 with a cut off of above 22–28 μg/mL or at Week 14135) above 16.55 μg/mL predicted a higher likelihood of sustained response over the first year. In regard to clinical remission one study identified that corticosteroid free, clinical and biochemical remission was correlated to higher trough vedolizumab concentration.136“
- Vedolizumab in younger patients: “Children under 30 kg require vedolizumab doses of 200 mg/m2 or 10 mg/kg.”
My take: “This NASPGHAN position paper should also serve to document that high-dose therapy, especially guided by TDM, is evidence-based standard of care.” This article clearly establishes three key points:
- “Intensive anti-TNF⍺ dosing strategies are not experimental. The initial doses of infliximab and adalimumab approved by the United States Food and Drug Administration (FDA) routinely lead to under-treatment, poor outcomes, and treatment discontinuation.60, 117 There is a rich, corroborated, and verified evidence-base to support the safety and efficacy of high-dose therapy anti-TNF⍺ therapy when clinically indicated, especially as supported by TDM.50, 62, 65, 100, 101, 103, 118“
- Therapeutic drug monitoring is essential in the pediatric population to optimize drug levels, allow many patients to do well with monotherapy, and to help avoid development of antidrug antibodies.
- The best available evidence supports TDM during induction of vedolizumab as well.
Related blog posts:
- Infliximab: Reach Higher and Be Stronger
- Another Study Justifying Higher Infliximab Dosing in Pediatrics
- Proactive Therapeutic Drug Monitoring in Pediatric Crohn’s disease -Better Outcomes | gutsandgrowth
- Is Standard Infliximab Dose Too Low in Pediatrics?
- Can Therapeutic Drug Monitoring with Monotherapy Achieve Similar Results as Combination Therapy for IBD? | gutsandgrowth
- Disease extent and need for higher infliximab dosing
- Proactive Therapeutic Drug Monitoring -Different Time Points | gutsandgrowth
- For the Next Insurance Appeal: Therapeutic Drug Monitoring in Adalimumab Treatment (Pediatrics) & Satire on Prior Authorizations | gutsandgrowth
- “Denials, Dilly-dallying and Despair”
- Kids Are Different: Therapeutic Drug Monitoring
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.