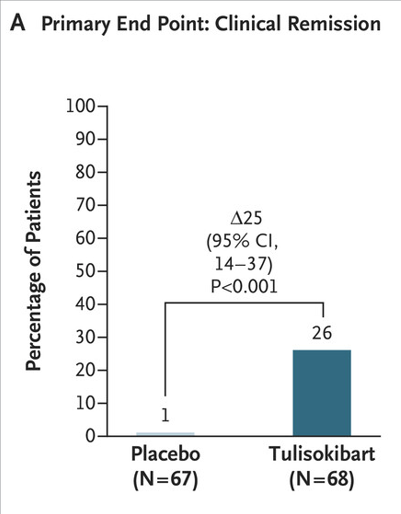
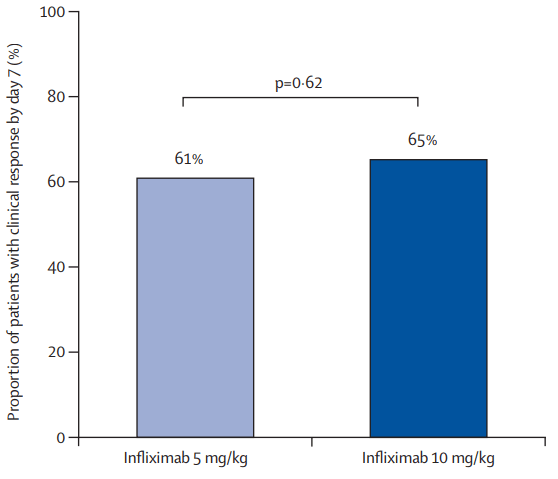
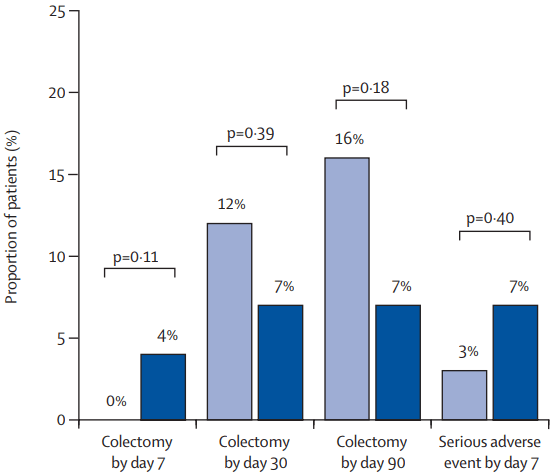
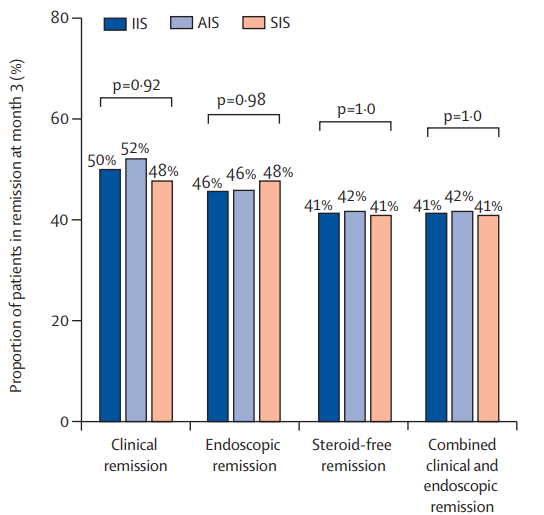
A Pudipeddi et al. Clin Gastroenterol Hepatol 2024; 22: 2299-2308. Open Access! Effects of Thiopurine Withdrawal on Vedolizumab-Treated Patients With Ulcerative Colitis: A Randomized Controlled Trial
Methods: This was a multicenter randomized controlled trial recruited UC patients (n=62) on vedolizumab 300 mg intravenously every 8 weeks and a thiopurine. Patients in steroid-free clinical remission for ≥6 months and endoscopic remission/improvement (Mayo endoscopic subscore ≤1) were randomized 2:1 to withdraw or continue thiopurine.
Key findings:
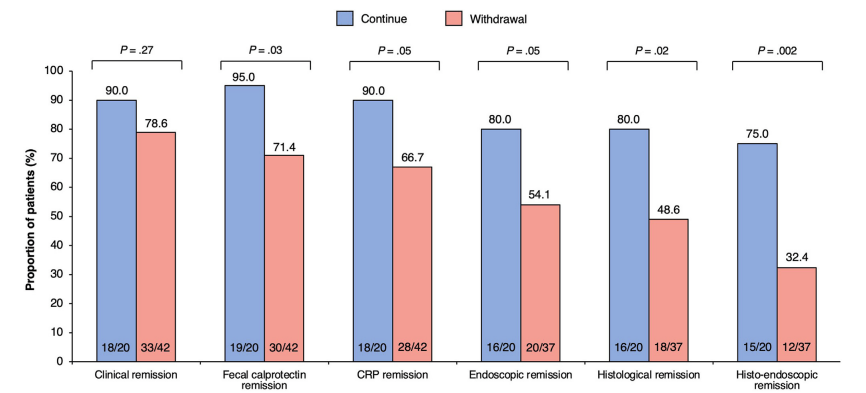
- At week 48, vedolizumab trough concentrations were not significantly different between continue and withdrawal groups (14.7 μg/mL versus 15.9 μg/mL, respectively, P = 0.36).
- The continue group had significantly higher fecal calprotectin remission (calprotectin <150) (95.0%, 19/20 versus 71.4%, 30/42; P = .03), histologic remission (80.0%, 16/20 versus 48.6%, 18/37; P = .02), and histo-endoscopic remission (75.0%, 15/20 versus 32.4%, 12/37; P = .002) than the withdrawal group. Clinical and endoscopic remission favored the continue group though this did not reach statistical significance.
- Histologic activity (hazard ratio [HR], 15.5; 95% confidence interval [CI], 1.6–146.5; P = .02) and prior anti-tumor necrosis factor exposure (HR, 6.5; 95% CI, 1.3–33.8; P = .03) predicted clinical relapse after thiopurine withdrawal.
Discussion: “In Australia, requirements are for UC patients to have failed at least 3 months of an immunomodulator before vedolizumab initiation. Consequently, UC patients are typically on combination therapy initially, and hence this study was designed as a withdrawal trial.” The authors note that previous studies have not shown superior outcomes with combination therapy (See blog post: No Benefit of Combination Therapy with Ustekinumab or Vedolizumab). “However, methodological flaws, heterogenous outcomes, and shorter durations of treatment limit these findings.”
My take (borrowed from authors): “Thiopurines might provide an incremental benefit to patients with UC using vedolizumab, … independent of vedolizumab pharmacokinetics.”
Related study: C Yzet et al. Clin Gastroenerol Hepatol 2021; 19: 668-679. Full Text: No Benefit of Concomitant Immunomodulator Therapy on Efficacy of Biologics That Are Not Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Diseases: A Meta-analysis
Related blog posts:
- COMBO-IBD Study -Combination Immunomodulator Use and Thresholds
- No Benefit of Combination Therapy with Ustekinumab or Vedolizumab
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.