7/1/24 FDA approves biosimilar Pyzchiva® (ustekinumab-ttwe), to be commercialized by Sandoz in US


N Sengupta et al. Am J Gastroenterol 2024; 119: 438-449. Open Access! The Role of Imaging for Gastrointestinal Bleeding: Consensus Recommendations From the American College of Gastroenterology and Society of Abdominal Radiology. Thanks to Dr. Benjamin Gold for this reference.
This article was jointly published: Radiology 2024; 310(3):e232298
This article focuses on GI bleeding in adults; it has a lot of useful information about the advantages, disadvantages, techniques and performance date of numerous radiology tests which can help sort out GI bleeding.
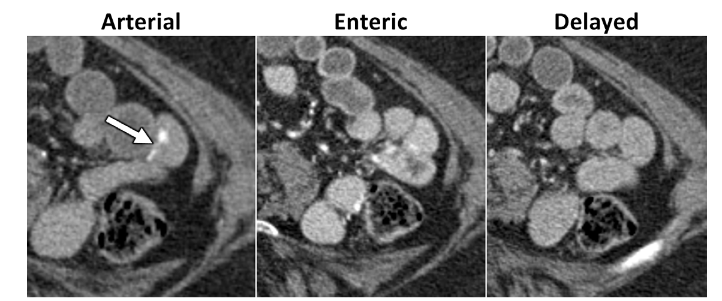

Some of the recommendations for Overt Lower GI Bleeding:
CT Angiography:
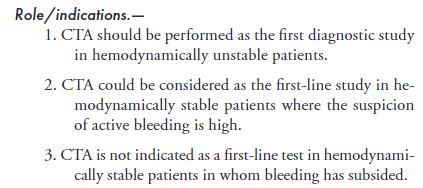
Catheter Angiography:

99mTc-RBC Scan

For Suspected Small Bowel Bleeding:
CT Enterography (uses oral contrast). Technique: CTE should be performed using multiphase technique in patients older than 40 years of age where vascular lesions are a common cause for bleeding.
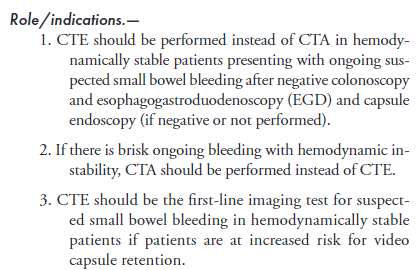
Meckel’s Scan “A Meckel scan can be considered to identify the cause of unexplained intermittent GI bleeding in children and adolescents after negative endoscopic evaluation, including capsule endoscopy if available, and cross-sectional evaluation of the small bowel.”
Radiology compared to capsule endoscopy and balloon-assisted endoscopy The authors discuss the advantages and limitations of radiologic testing versus capsule endoscopy and balloon-assisted endoscopy for small bowel bleeding is provided in Appendix S5
My take: This article provides a good update/review on useful radiologic imaging for GI bleeding. For pediatric GI bleeding, the etiologies are much different and many patients should be evaluated with a Meckel’s scan prior to panendoscopy (depending on the clinical presentation).
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
R Michael, J Tozer. N Engl J Med 2024;390: e53. Small-Bowel Intussusception in an Adult
The authors describe the presentation/resolution of a 57 yo with a small bowel intussusception due to a fibroblastic polyp lead point.
Video: Reduction of Intussusception (may be behind a paywall)

Related blog posts:
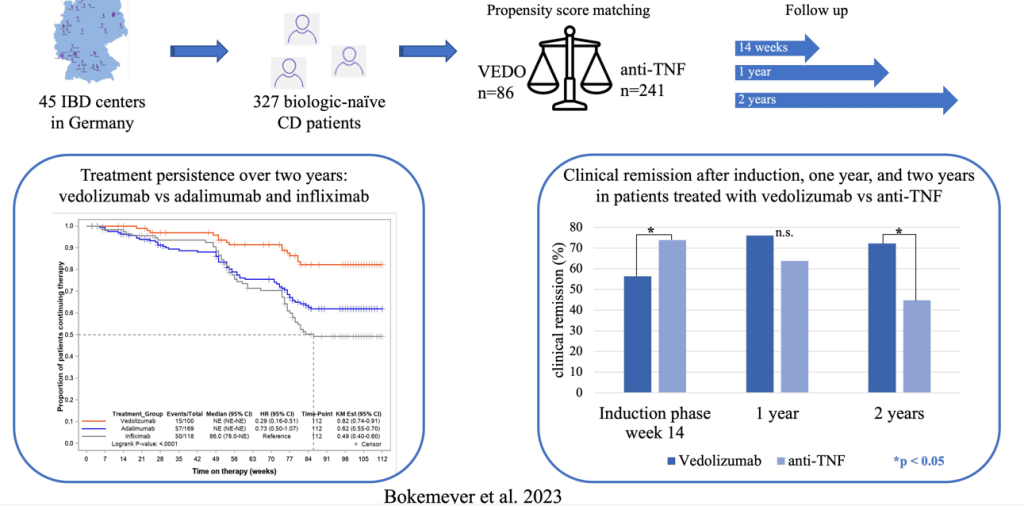
B Bokemeyer et al. Inflamm Bowel Dis 2024; 30: 746-756.
Methods: 3277 adult biologically-unexposed CD patients starting therapy with VEDO or anti-TNF were consecutively enrolled in 45 IBD centers across Germany (2017-202). This was a non-randomized, observational study with prospectively collected data.
Findings:
The discussion describes the strengths and limitations of this study. As it is not a randomized control trial, there can still be selection bias and confounding even with propensity scoring that was done in this study. The authors note that in a prior analysis of RCTs comparing infliximab to vedolizumab in CD patients, that infliximab had higher efficacy for induction and maintenance, though the clinical remission rates were only modestly improved at 1 year. (L Peyrin-Biroulet et al. BMC Gastroenterol 2022; 22: 291).
Recent expert guidance (2024) has favored infliximab and risankizumab over other advance therapies in CD patients who have not had previous biologic therapies (see: Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease).
My take: This study shows that vedolizumab is a good advanced therapy for patients with Crohn’s disease without prior therapy. Among those with a clinical response at 14 weeks, the treatment durability was particularly impressive in this cohort.
It would be great to see an RCT in children with CD comparing IFX to VEDO. Treatment persistence is even more important in younger patients.
Related blog posts:
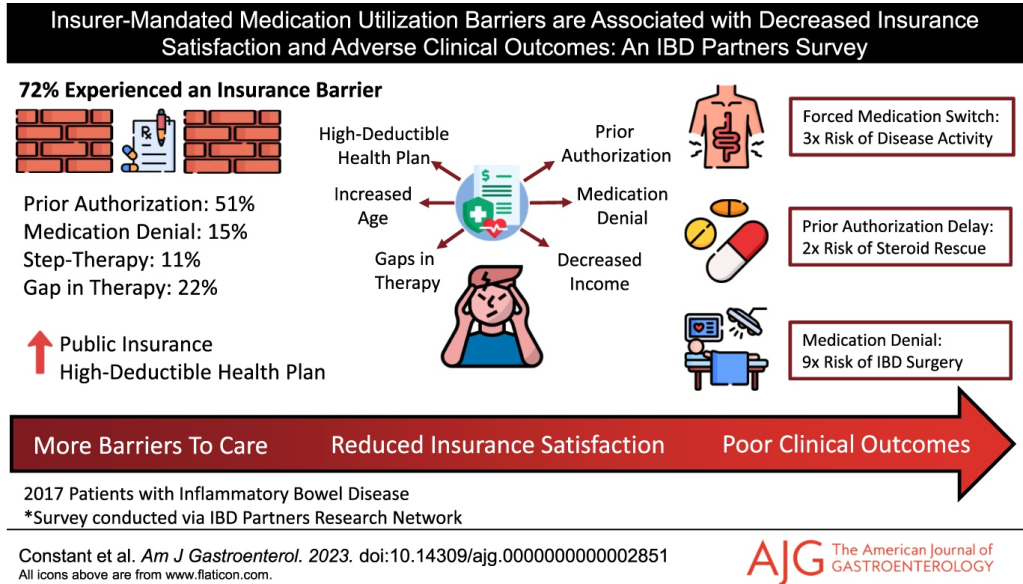
B Constant et al. AJG 2024; DOI: 10.14309/ajg.0000000000002851. Insurer-Mandated Medication Utilization Barriers are Associated With Decreased Insurance Satisfaction and Adverse Clinical Outcomes: An Inflammatory Bowel Disease Partners Survey
Key findings: In this longitudinal survey with 2017 patients, 72% experienced an insurer-mandated barrier, most commonly prior authorizations (51%). Fifteen percent were denied an IBD medication by their insurer, 22% experienced an insurance-related gap in therapy, and 8% were forced by their insurer to switch from an effective medication. Several insurance barriers were linked to negative downstream clinical outcomes, including prior authorizations associated with corticosteroid rescue (odds ratio [OR] 2.24]), forced medication switches associated with continued disease activity (OR 3.28), and medication denials associated with IBD-related surgery (OR 8.92).
Related blog posts:
S Danese et al. Lancet Gastroenterol Hepatol 2024; 9: 133-146. Efficacy and safety of 48 weeks of guselkumab for patients with Crohn’s disease: maintenance results from the phase 2, randomised, double-blind GALAXI-1 trial
In this phase 2 randomised, multicentre, double-blind trial with 309 adults, the authors report on the safety and efficacy of subcutaneous guselkumab maintenance regimens to week 48 in the GALAXI-1 study. Key findings:
Related blog posts:
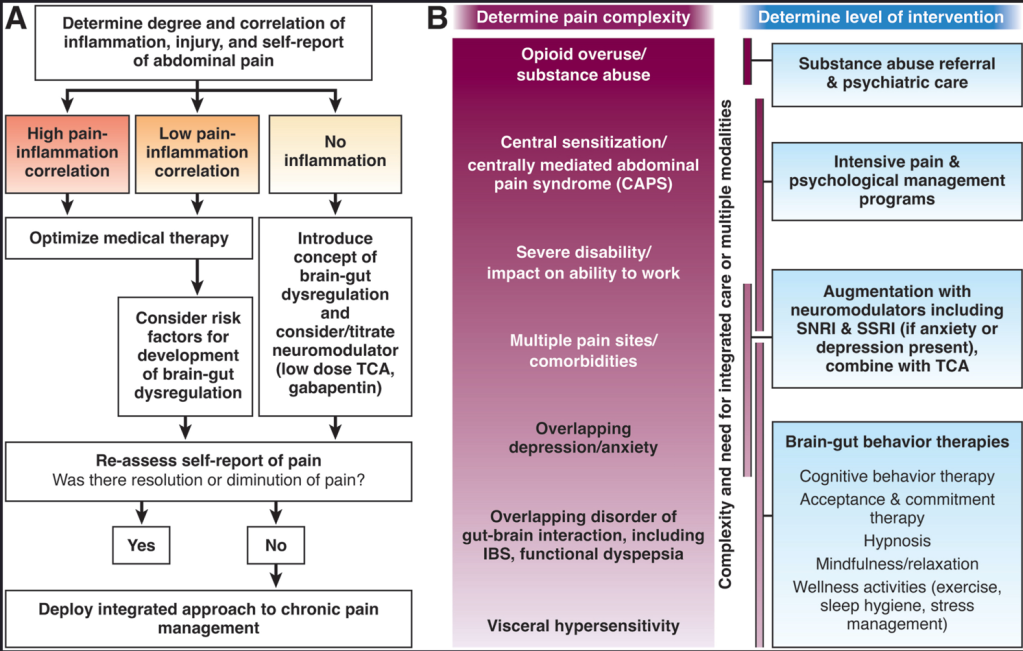
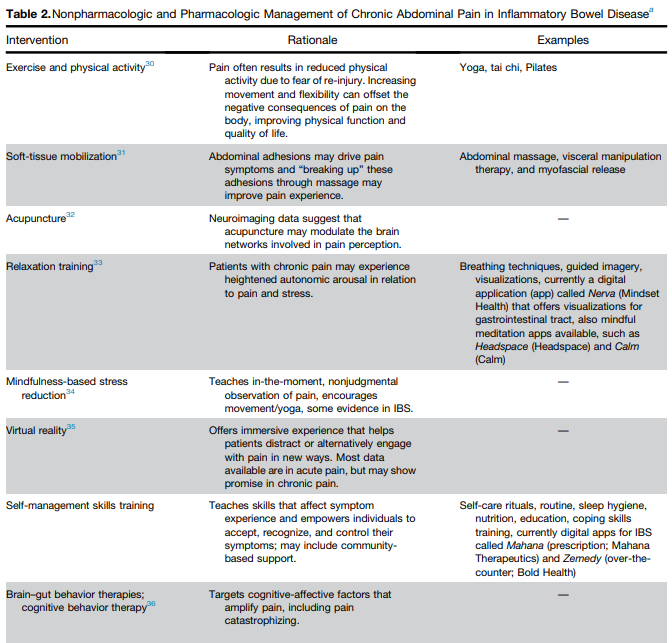
L Keefer et al. Gastroenterology 2024; 166: 1182-1189. AGA Clinical Practice Update on Pain Management in Inflammatory Bowel Disease: Commentary
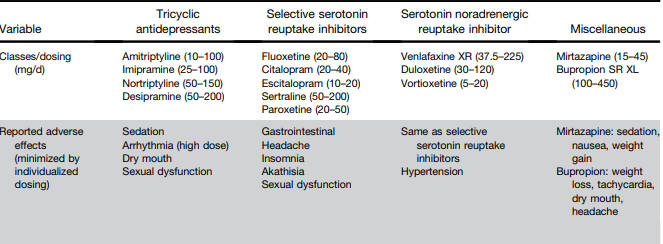
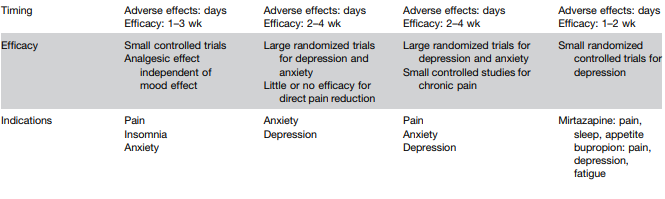
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
A Rubio-Tapia et al. Gastroenterol 2024; 166: 930-934. Open Access! AGA Clinical Practice Update on Diagnosis and Management of Cannabinoid Hyperemesis Syndrome: Commentary
Key points:
My take: The most cyclical (cynical) part of CHS is the stereotypical attempt to get patients to stop using cannabis.
As an aside, cannabis use is often disclosed by patients having procedures and their atypical response to sedation/anesthesia. Use of anesthetics including propofol can have cross-reactivity with cannabis. In addition, several studies have demonstrated that cannabis users are more likely to report higher pain scores, poorer sleep, and require a greater quantity of analgesic medications in the immediate postoperative period than nonusers.
Related blog posts:
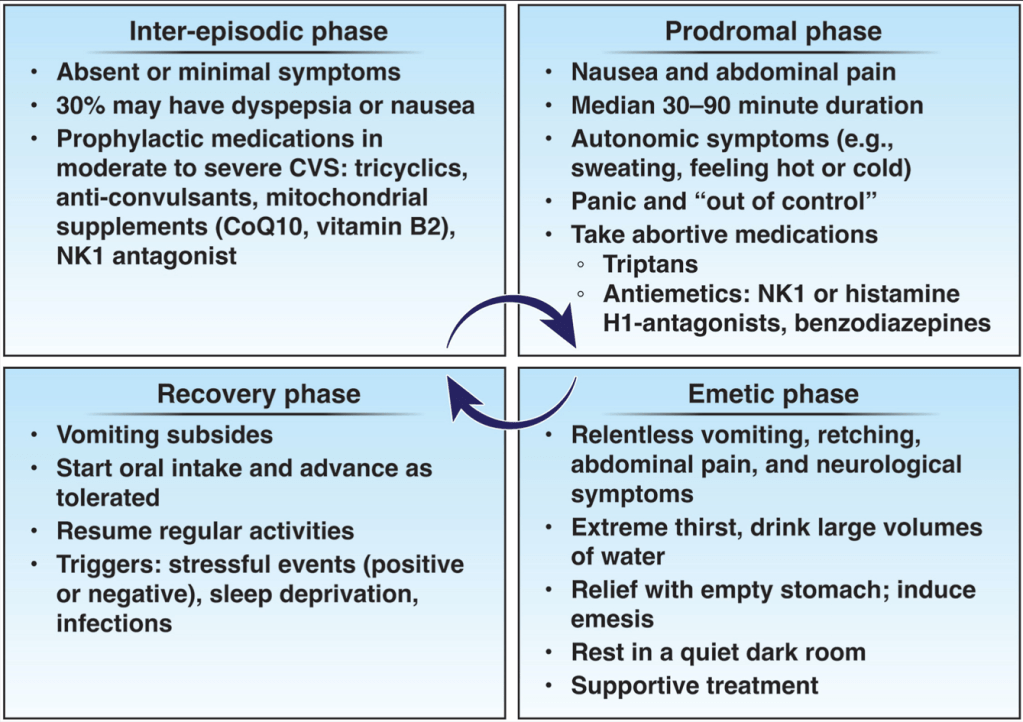
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
C Hernandez-Rocha et al. J Crohns Colitis 2024: 18: 615–627. Clinical Predictors of Early and Late Endoscopic Recurrence Following Ileocolonic Resection in Crohn’s Disease
Prospective Study n=365 adult patients with post-operative Crohn’s disease. Findings: At first colonoscopy, 109 [29.9%] had recurrence. Male gender (odds ratio [OR] = 1.95), non-White ethnicity [OR = 2.48], and postoperative smoking [OR = 2.78] were associated with recurrence, while prophylactic anti-TNF reduced the risk [OR = 0.28]. Postoperative anti-TNF prophylaxis had a protective effect on anti-TNF experienced patients but not on anti-TNF naïve patients. Among patients without recurrence at first colonoscopy, Rutgeerts score i1 was associated with subsequent recurrence [OR = 4.43]
A Lecoutour et al JPGN 2024; 78:1116–1125. Efficacy of infliximab after loss of response of/intolerance to adalimumab in pediatric Crohn’s disease: A retrospective multicenter cohort study of the “GETAID pédiatrique”
Key findings: In this retrospective study, 27 of 32 patients (84.4%) were still on IFX at 12 months of the switch. Among them, 13 had discontinued ADA because of a LOR, 12 for insufficient response and 2 due to primary nonresponse. At 1 year, 22 patients were in corticosteroid free clinical remission (68.7%).

PV Patel et al. JPGN 2024; 78:1126–1134. Real‐world effectiveness of ustekinumab and vedolizumab in TNF‐exposed pediatric patients with ulcerative colitis
Using the ICN registry, this observational study had 262 anti-TNF refractory patients receiving VDZ and 74 patients receiving UST. Key finding: At 6 months, 28.3% of patients on VDZ and 25.8% of those on UST achieved CFCR (p= 0.76)


PS Dulai et al. Gastroenterol 2024; 166: 396-408. Open Access! Integrating Evidence to Guide Use of Biologics and Small Molecules for Inflammatory Bowel Diseases
“In this review, we provide a framework for clinicians and researchers to understand key differences in sources of evidence, how different methodologies are applied to study the comparative effectiveness of advanced medical therapies in IBD, and considerations for how these sources of evidence can be used to better integrate current guideline recommendations.”
This article explains the use of randomized controlled trials, “real-world evidence”/observational comparative studies, network meta-analysis, and post-hoc comparisons from randomized studies.
“The authors advocate for “”Given the rapidity with which new advanced medical therapies are becoming available in IBD, which quickly make current guidelines obsolete, living guidelines may offer a unique consideration to ensure applicability to routine care.”
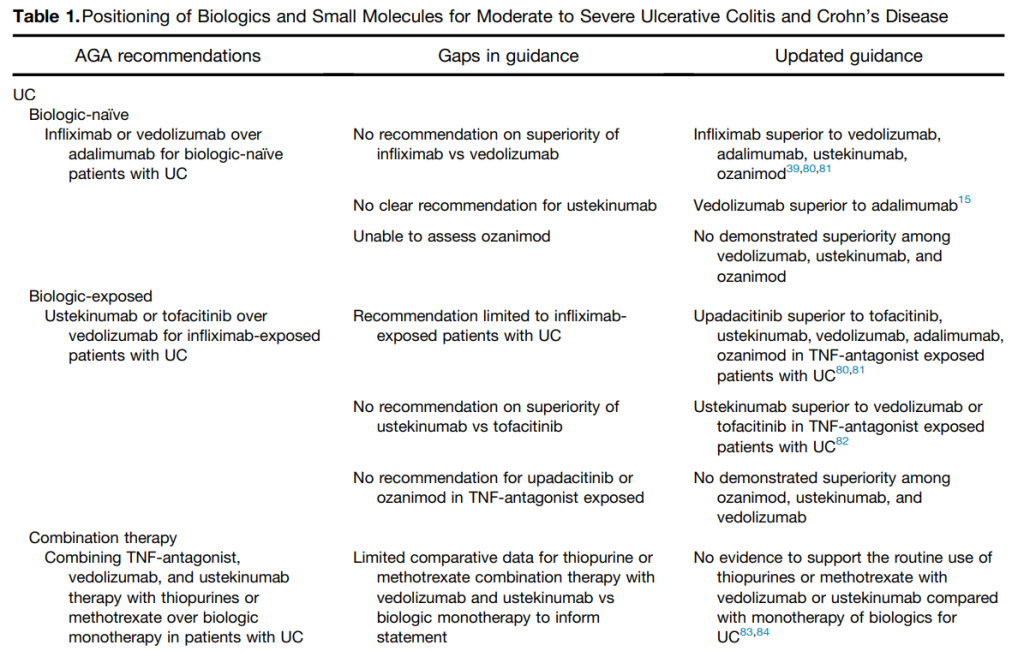

My take: This article provides a useful update of current advanced therapies and information in positioning these advanced therapies. It would be a great service if the IBD community could create something similar to HCVguidelines.org. The latter was a coordinated effort by the AASLD and IDSA to help provide expert advice during a deluge of amazing advances in HCV. And just like HCVguidelines, it is important to address “special” populations including pediatric patients and patients with very early onset IBD.
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
R Lujan, R Buchuk et al. Gastroenterol 2024; 166: 815-825.Early Initiation of Biologics and Disease Outcomes in Adults and Children With Inflammatory Bowel Diseases: Results From the Epidemiology Group of the Nationwide Israeli Inflammatory Bowel Disease Research Nucleus Cohort
Methods: All patients diagnosed with CD or UC in Israel (2005–2020) were included in the Epidemiology Group of the Israeli Inflammatory Bowel Disease Research Nucleus cohort. The authors compared disease duration at biologics initiation (ie, 0–3 months, >3–12 months, >1–2 years, and >2–3 years) using the cloning, censoring, and weighting by inverse probabilities method to emulate a target trial, adjusting for time-varying confounders and selection bias.
Of the 34,375 included patients (of whom 5240 [15%] were children), 7452 of 19,264 (39%) with CD and 2235 of 15,111 (15%) with UC received biologics. To attempt to adjust for patient characteristics, “essentially, each patient was cloned 4 times and 1 clone was assigend to each treatment strategy at baseline…[they were removed] subsequently if they did not receive the biologic within the acceptable time window.” Key findings:
Thus, overall, the study found that early initiation of biologics was associated only with a modest reduced risk of surgery and steroid dependency for Crohn’s disease. It was not found to reduce risk of colectomy or steroid dependency in UC.
My take: In my view, this study probably underestimates the benefits of early biologic therapy. Even though, lead time bias can skew interpretation, inadequately-treated disease likely leads to long-term damage. The associated editorial (pg 728) by Murphy notes that despite the sophisticated statistical methodology, all observational studies cannot fully control for unmeasured confounders.
Related blog posts:

This past weekend there were two fun events in Atlanta -Porchfest (Virginia Highlands) and Midtown Garden Stroll. Porchfest featured more than 50 local bands.
Here’s a couple photos from the Midtown Garden Stroll:





IK Araujo et al. Clin Gastroenterol Hepatol 2024; 22: 513-522. The Severity of Reduced Esophageal Distensibility Parallels Eosinophilic Esophagitis Disease Duration
This study of 171 adult patients (mean age 38 years) who had FLIP at time of an EGD determined the degree of esophageal distensibility and its association with eosinophilic esophagitis disease duration.
Key findings:

My take: Longer duration of disease increases the risk of esophageal fibrosis and lack of distensibility. We need better tools to predict who is at most risk for developing fibrosis.
Related blog posts: