B Wang et al. Gastroenterol 2023; 164: 484-491. Open Access! AGA Clinical Practice Update on Diagnosis and Management of Acute Hepatic Porphyrias: Expert Review
Overall, acute hepatic porphyrias (AHP) are rare disorders. “Acute intermittent porphyria is the most common type of AHP, with an estimated prevalence of patients with symptoms of
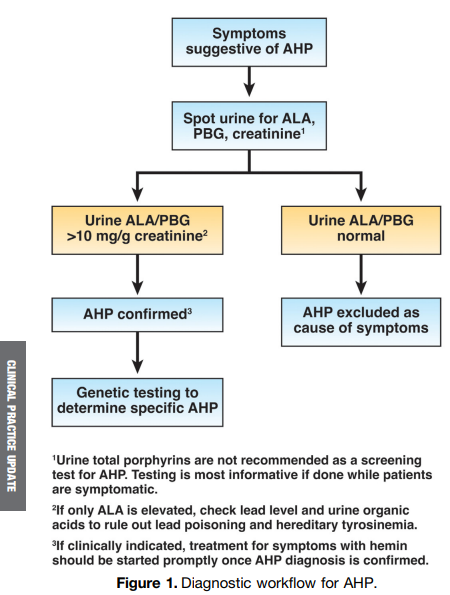
approximately 1 in 100,000. The major clinical presentation involves attacks of severe pain, usually abdominal and generalized, without peritoneal signs or abnormalities on cross-sectional imaging…The screening tests of choice include random urine porphobilinogen and d-aminolevulinic acid corrected to creatinine.”
“All patients with elevations in urinary porphobilinogen and/or d-aminolevulinic acid should initially be presumed to have AHP. The cornerstones of management include discontinuation of porphyrinogenic drugs and chemicals, administration of oral or intravenous dextrose and
intravenous hemin, and use of analgesics and antiemetics. Diagnosis of AHP type can be confirmed after initial treatment by genetic testing for pathogenic variants in HMBS, CPOX, PPOX, and ALAD genes.”
Some of the best practice advice:
- Women aged 15–50 years with unexplained, recurrent severe abdominal pain without a clear etiology after an initial workup should be considered for screening for an AHP.
- Initial diagnosis of AHP should be made by biochemical testing measuring d-aminolevulinic acid, porphobilinogen, and creatinine on a random urine sample.
- Genetic testing should be used to confirm the diagnosis of AHP in patients with positive
biochemical testing. - Acute attacks of AHP that are severe enough to require hospital admission should
be treated with intravenous hemin, given daily, preferably into a high-flow central vein
Related blog posts: