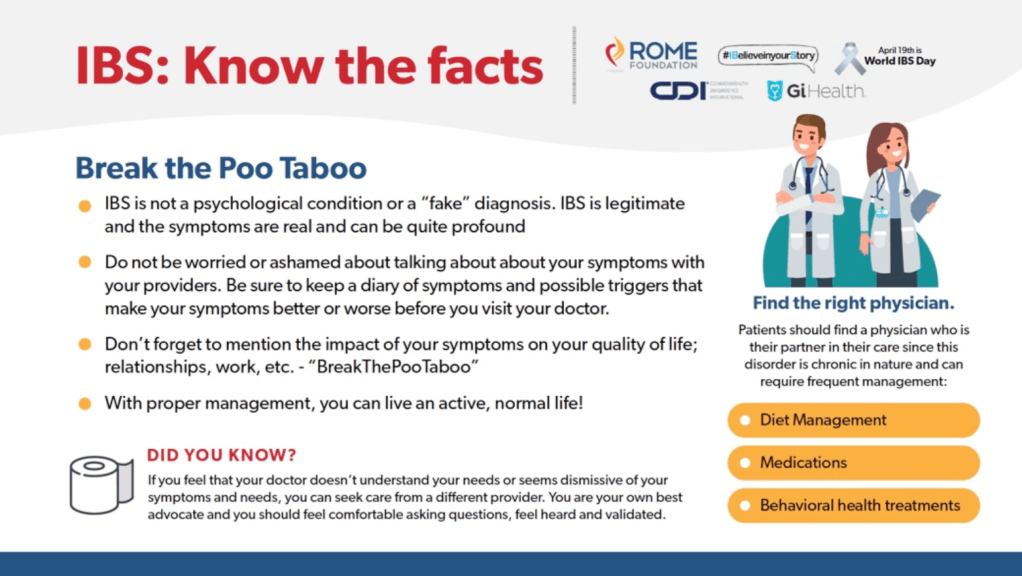
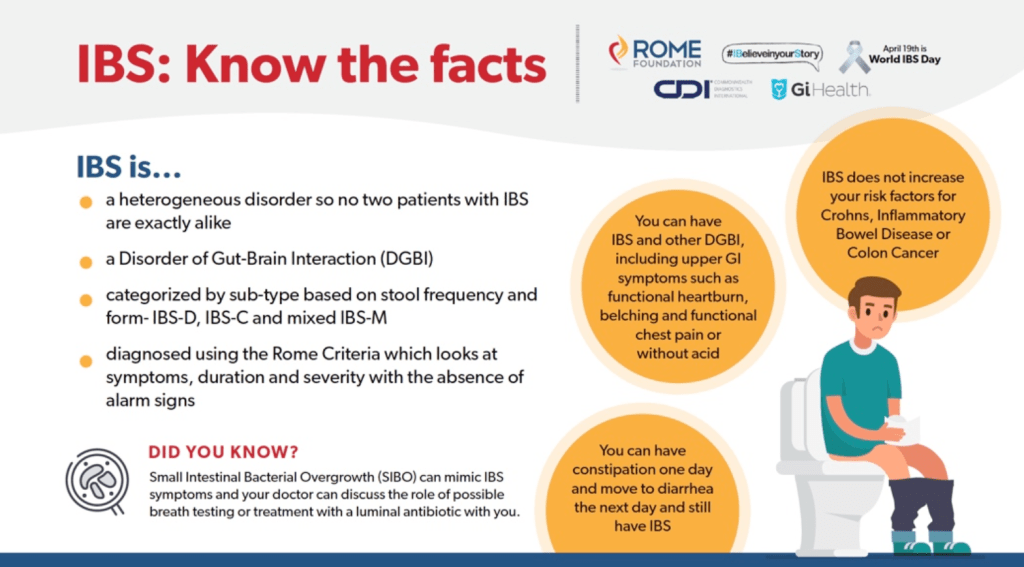
Briefly noted: RK Goyal. NEJM 2021; 384: 1742-1751. Gastric Emptying Abnormalities in Diabetes Mellitus
This article provides insight into the topic of gastric emptying with a focus on patients with diabetes. A few key points:
- Gastric emptying affects glucose homeostasis in patients with diabetes; delayed gastric emptying in patients with type 2 diabetes could have beneficial effects in this regard.
- Delayed gastric emptying occurs in 40-47% of adults with diabetes; rapid emptying occurs in 20-22%.
- Upper GI symptoms do NOT correlate with gastric emptying. Prevalence of these symptoms is highest in those with normal gastric emptying (43-52% in those with normal emptying compared with 19-28% with delayed emptying, and 20-37% with rapid emptying)
- “Functional dyspepsia-like symptoms in gastroparesis may arise not through motility changes but rather through the parallel effects of oxidative stress and inflammation on nocireceptors and on other afferents that produce the symptoms.”
My take: Knowing how quickly the stomach empties rarely helps management. In this review, Dr. Goyal states that “the effective treatment of symptoms in diabetic gastroparesis may be similar to the treatment of functional dyspepsia.”
Also, noted in same issue of NEJM:
TB Corcoran et al. NEJM 2021; 384: 1731-1741. Dexamethasone and Surgical-Site Infection Key finding: A single dose of dexamathosone (8 mg) did not increase the risk of surgical site infection; this is in contrast to long-term glucocorticoid therapy which is a risk factor for infection and wound dehiscence.
J Salwa et al. NEJM 2021; 384: 1684-6. Designing an Independent Public Health Agency. This article makes compelling arguments for separating health agencies from political influence. The FDA, the CDC, and HHS in the previous administration were pressured and undermined. In contrast, the Federal Reserve Board, which has 14 year terms that require ‘removal only for cause,’ was “reliably [able to] exert federal power because of its institutional features as an independent agency.”
From TikTok -twitter feed: The GI Bleeding Paradox (58 secs -humor) @DGlaucomflecken#Gastroenterology#GI#MedTwitter