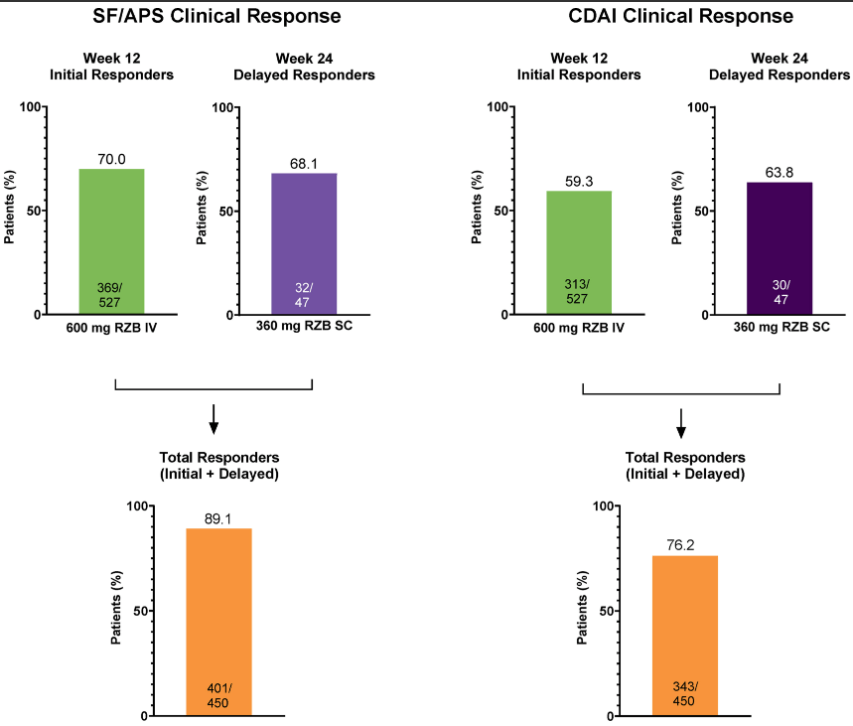
- VB Young. NEJM 2025; 392: 1237-1240. Vaccinating against Clostridioides difficile Infection
- MG Alameh et al. Science 2024;386:69-75. A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection
This NEJM review describes recent developments in vaccines for C difficile.
Background: “Because the pathogenesis of C. difficile infection depends on the production of the potent toxins TcdA (toxin A) and TcdB (toxin B) by vegetative C. difficile cells, there is hope that the development of vaccines targeting these virulence factors…will be successful in limiting the development of C. difficile infection in patients receiving antibiotic treatment.2“
An excerpt:
“A trial of a vaccine composed of formalin-inactivated TcdA and TcdB purified from a highly toxigenic C. difficile strain was stopped at the first planned interim analysis on the basis of clinical futility,3 and the development of this vaccine was terminated. More recently, results were published from the Clostridium difficile Vaccine Efficacy Trial (CLOVER), a phase 3, randomized trial of a genetically detoxified C. difficile vaccine composed of recombinant TcdA and TcdB (containing targeted amino-acid substitutions to limit toxic activity) that were further detoxified by chemical means.4 Although the trial did not show a benefit with respect to the primary end point of preventing a first episode of C. difficile infection, vaccinated patients in whom C. difficile infection developed had a shorter duration of symptoms and were less likely to receive medical attention for their infection than patients who had received placebo.
In this setting, a new type of C. difficile vaccine candidate, described by Alameh, Semon, and colleagues,5 is of interest. These investigators developed a multivalent nucleoside-modified messenger RNA (mRNA) vaccine (see Key Concepts) delivered in lipid nanoparticles (LNPs)…The mRNA–LNP vaccine elicited higher antibody levels to all three vaccine targets than the recombinant vaccine with alum adjuvant. Furthermore, the mRNA–LNP vaccine provided complete protection against challenge with an intraperitoneally administered high dose of purified TcdA or TcdB: all the vaccinated mice survived, whereas all the unvaccinated mice were moribund within 2 days. The recombinant–alum vaccines protected only 20% of the vaccinated animals…
However, protection was not associated with the prevention of colonization: all the vaccinated animals shed high numbers of culturable C. difficile and had histopathological damage to intestinal tissue that was equivalent to that seen in unvaccinated animals according to analyses performed 2 days after infection. This finding suggests that protection was due to blocking of the systemic effects of the C. difficile toxins. However, additional data indicated that inclusion of the PPEP-1 antigen in the multivalent vaccine resulted in more rapid clearance of luminal toxin levels.”
My take: An effective vaccine would be a welcome advance and perhaps limit the shitty treatments we have had to date.
Related blog posts:
- OpenBiome Suspending FMT Shipments
- Large Study Shows FMT Efficacy/Safety in Children
- FMT in the “Real World”
- Rejected! Most Stool is Not Good Enough for FMT