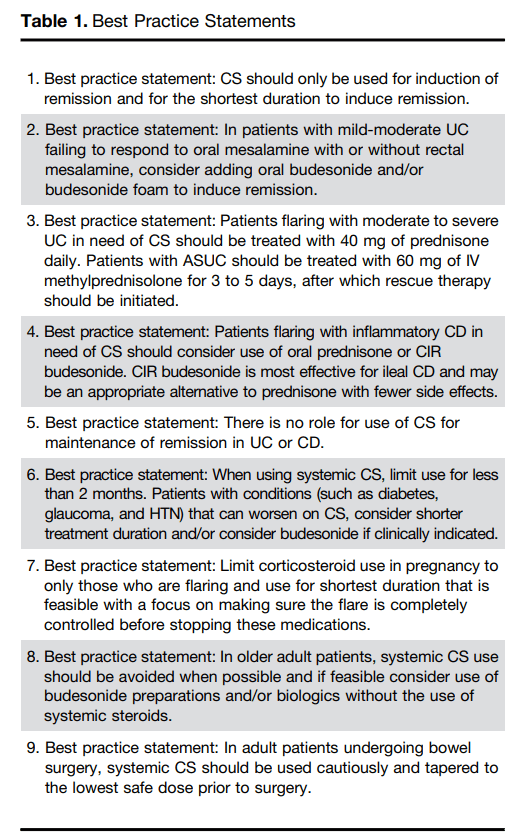
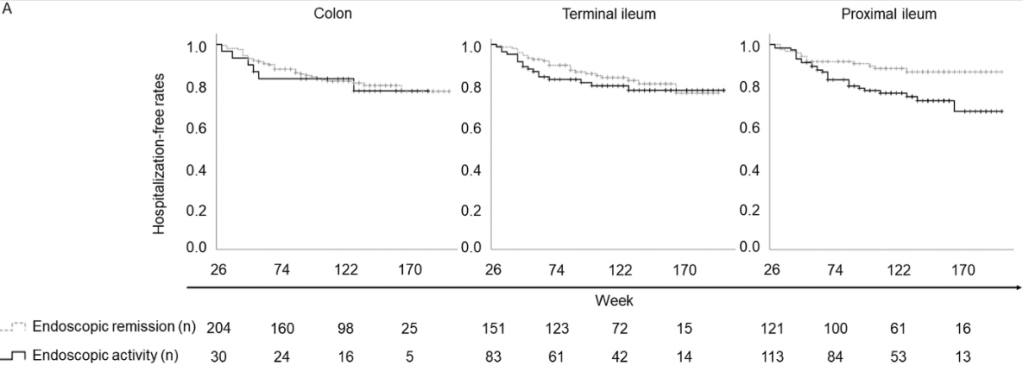
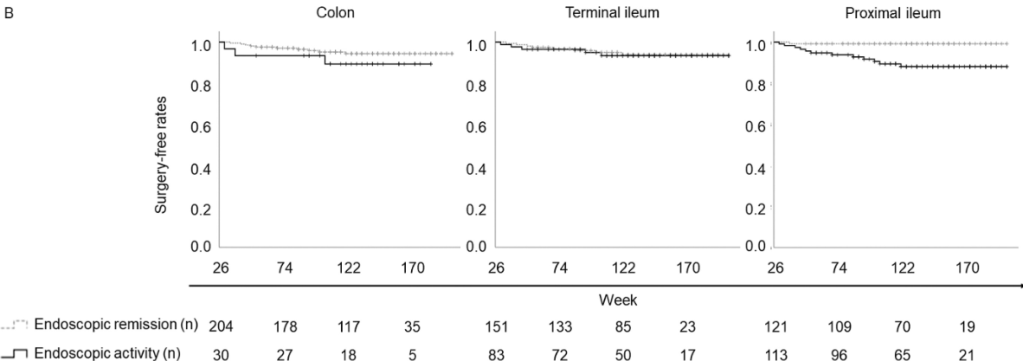
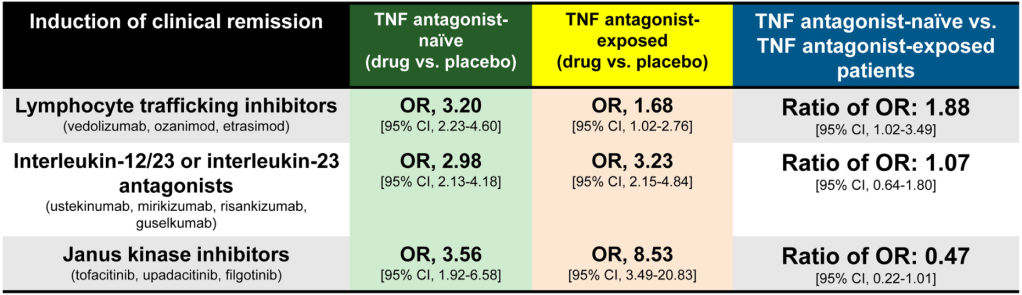
D Turner et al. . Gut 2025;0:1–7. doi:10.1136/gutjnl-2025-336368. Preclinical stages of Crohn’s disease defined by faecal calprotectin in asymptomatic first-degree relatives: screening framework for prevention trial
Methods: “Faecal calprotectin was measured in asymptomatic FDRs aged 6–38 years; those with persistent elevation, defined as >70 µg/g in at least two separate tests, were offered panenteric video capsule endoscopy or ileocolonoscopy”
Population: 331 (35%) first-degree relatives (FDRs) (from a group of 950) agreed to be screened: 63 (19%) had persistently elevated calprotectin, of whom 42 underwent further evaluation
Key findings:
- From the initial screened cohort of 331 patients, nine (2.7%) had endoscopic appearance compatible with presymptomatic CD, and 22 (6.6%) had non-specific macroscopic mucosal changes
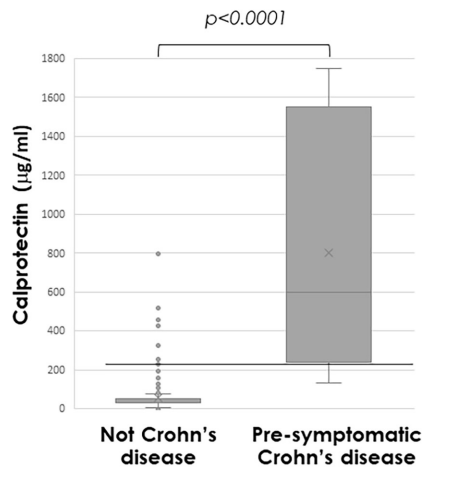
- Median calprotectin was significantly higher in those with presymptomatic CD (772µg/g (IQR 279–1685)) compared with others (31µg/g (IQR 30–61), p<0.0001)
- Calprotectin >225 µg/g predicted presymptomatic CD (area under the receiver operating
characteristic curve 0.97 (95% CI 0.94 to 1.0; p<0.001; sensitivity 89%, specificity 94%)
Discussion Points:
- “There is no universally accepted definition for preclinical stages of CD, and
the distinction between these stages remains partly subjective.” - “The lack of longitudinal follow-up is also a limitation, but this will be completed as part of the PIONIR trial.”
My take (borrowed in part from the authors):
- Identification of pre-symptomatic CD “can facilitate designing targeted interventions and defining inclusion criteria for prevention trials.” The disease may be more modifiable in the early stages of disease.
- This trial suggests the calprotectin threshold of >70 is too low to target screening. For specificity, the study showed that persistent elevation above 225 merits investigation; though, it has been our practice to use a threshold of >150 for children older than 5 years.
- Approximately 5% of asymptomatic FDRs of CD patients have evidence of pre-symptomatic CD and approximately 10% more have non-specific mucosal changes when evaluated
Related blog posts:
- “Silent” Crohn’s Disease
- Mom, Can We Get a Dog (& a Sibling)? I Don’t Want to Get Crohn’s Disease
- Intestinal Barrier Function and Risk of Crohn’s Disease
- What is the Calprotectin Threshold for Disease Progression in Crohn’s Disease?
- Risk Factors for Inflammatory Bowel Disease: Ultra-Processed Food (Part 1)
- Ultraprocessed Food and the Risk of Inflammatory Bowel Disease
- Risk Factors for Inflammatory Bowel Disease: Antibiotics (Part 2)
- Early Antibiotics -Minimal Risk for Crohn’s Disease