DA Schwartz et al. Clin Gastroenterol Hepatol 2022; 20: 1059-1067. Open Access: Efficacy and Safety of 2 Vedolizumab Intravenous Regimens for Perianal Fistulizing Crohn’s Disease: ENTERPRISE Study
Methods: “Patients with moderately to severely active CD and 1–3 active perianal fistulae (identified on magnetic resonance imaging [MRI]) received vedolizumab 300 mg intravenously at weeks 0, 2, 6, 14, and 22 (VDZ) or the same regimen plus an additional vedolizumab dose at week 10 (VDZ + wk10)… Enrollment was stopped prematurely because of recruitment challenges”
Key findings:
- “Rapid and sustained fistula closure was observed; 53.6% (VDZ, 64.3%; VDZ + wk10, 42.9%) and 42.9% (VDZ, 50.0%; VDZ + wk10, 35.7%) of patients achieved ≥50% decrease in draining fistulae and 100% fistulae closure, respectively, at week 30”
- “MRI healing, defined as the disappearance of T2 hyperintensity signal and absence of gadolinium contrast enhancement,3 was not reached in this study…gadolinium contrast enhancement showed improvement at week 30…MRI studies have shown that internal fistulae healing lags behind clinical remission by a median of 12 months”
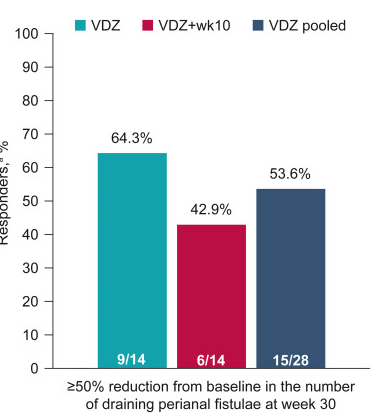
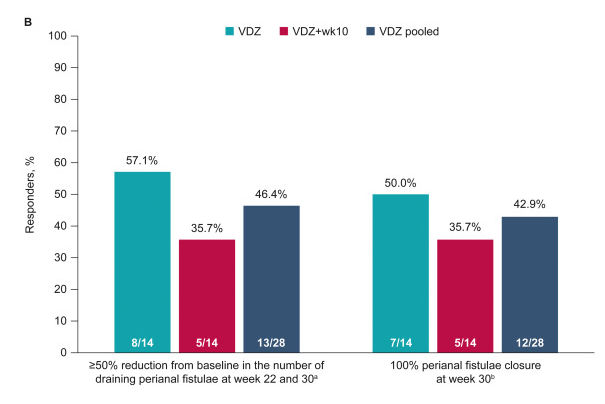
The study findings are limited by relatively small size and lack of control group (eg. placebo or seton/antibiotic group). However, the rate of response in this study is significantly higher than placebo studies which have shown “~1 in 6” who experienced fistula closure.
My take: Vedolizumab is another option for treating Crohn’s disease with perianal fistula. Both regimens in this study were associated with response, though the additional 10-week dose (in one group) did not improve outcomes.
Related blog posts:
- Ustekinumab Over Vedolizumab as 2nd Line Agent for Crohn’s Disease
- Comparative Efficacy: Vedolizumab vs Anti-TNF Agents
- “Positioning Biologic Therapies in the Management of Pediatric Inflammatory Bowel Disease” & 14% of U.S. Infected with COVID-19 | gutsandgrowth
- 2021 AGA Guidelines For Crohn’s Disease
- Expert Guidance on Current IBD Mgt (2020)
- Expert Guidance on Inflammatory Bowel Disease (Part 3)
- Expert Guidance on Inflammatory Bowel Disease (Part 2)
- Comparative Efficacy: Vedolizumab vs Anti-TNF Agents