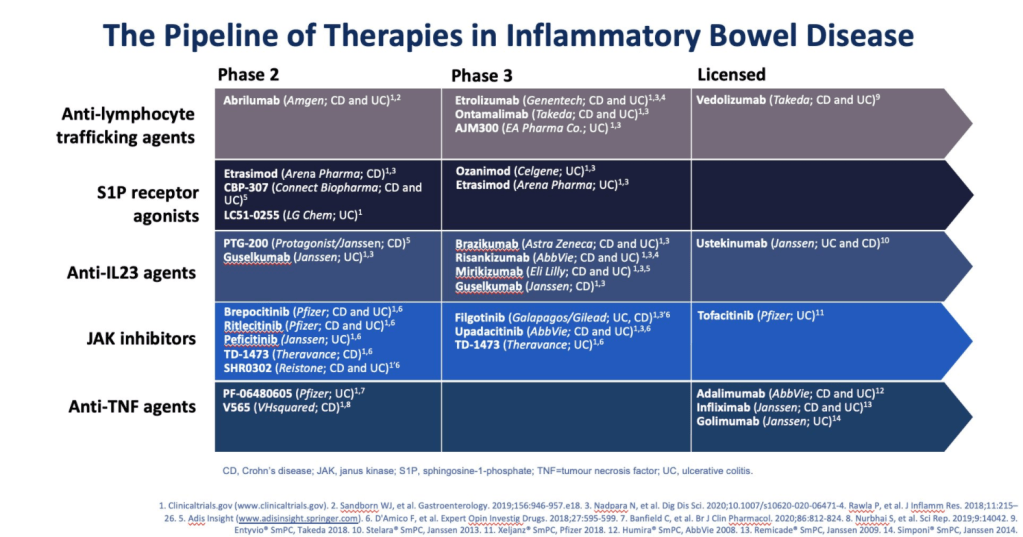
LC Shipley et al. Clin Gastroenterol Hepatol 2022; 20: 455-457. Vedolizumab Therapy in Refractory Microscopic Colitis: A Single Center Case Series
In this report, the authors describe nine patients with refractory microscopic colitis (median age 55 years) who were treated with vedolizumab.
Key findings:
- Clinical response with induction in 9 (100%); time to >50% response ranged from 1 to 7 weeks with 5 patients responding within 2 weeks.
- Sustained response with maintenance therapy in 6 (67%); duration of follow-up ranged from 1 month to 15 months. The three patients without response had symptom duration of 10 yrs, 12 yrs, and 25 yrs prior to institution of vedolizumab.
- Only two patients had histologic follow-up. While both had clinical response, the patient with lymphocytic colitis had histologic resolution whereas a patient with collagenous colitis had histologic persistent.
My take: Given vedolizumab’s favorable safety profile, further studies (with endoscopic endpoints) of vedolizumab are needed to define its efficacy for microscopic colitis.
Another study with vedolizumab: J Kirchgesner et al. Clin Gastroenterol Hepatol 2022; 20: 314-324. Risk of Serious Infections With Vedolizumab Versus Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Disease
Key finding: The risk of serious infections was not different between vedolizumab and anti-TNF in the overall IBD cohort (HR, 0.95; 95% CI, 0·79-1.13), while the risk was decreased for vedolizumab users in patients with UC (HR, 0.68; 95% CI, 0.50-0.93), but not CD (HR, 1.10; 95% CI, 0.87-1.38)
Related blog post/related article:
- How Many Biopsies Are Sufficient for Diagnosis of Microscopic Colitis?
- Can Microscopic Colitis Lead to UC or Crohn’s Disease?
- #NASPGHAN19 Postgraduate Course (Part 5)
- Full text PDF: AGA Microscopic Colitis Guideline: GC Nguyen et al. Gastroenterol 2016; 150: 242-6. Technical review DS Pardi et al. Gastroenterol 2016; 150: 247-74. Patient guide: pg 275.
- Microscopic, Lymphocytic and Collagenous Colitis
- Is Vedolizumab the Best First Line Biologic in Ulcerative Colitis?
- Comparative Efficacy: Vedolizumab vs Anti-TNF Agents
- Real-World Vedolizumab: Better Than Expected

Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.