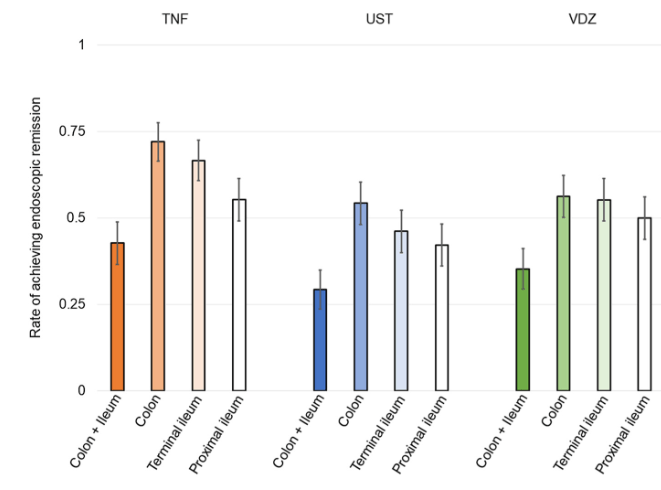
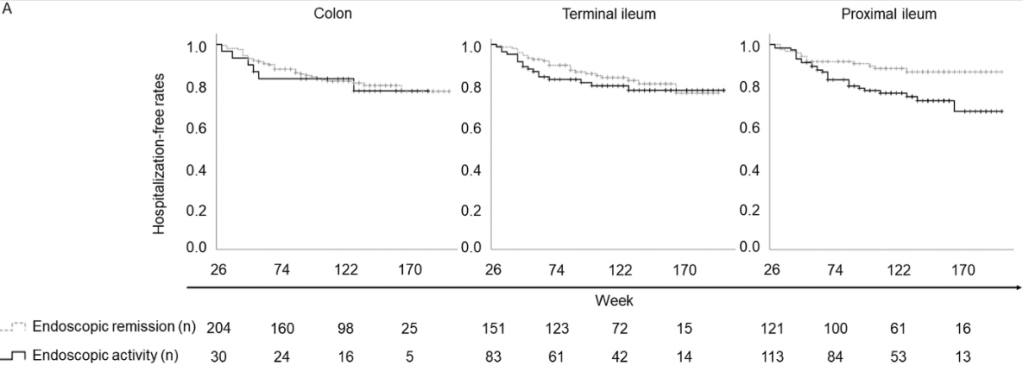
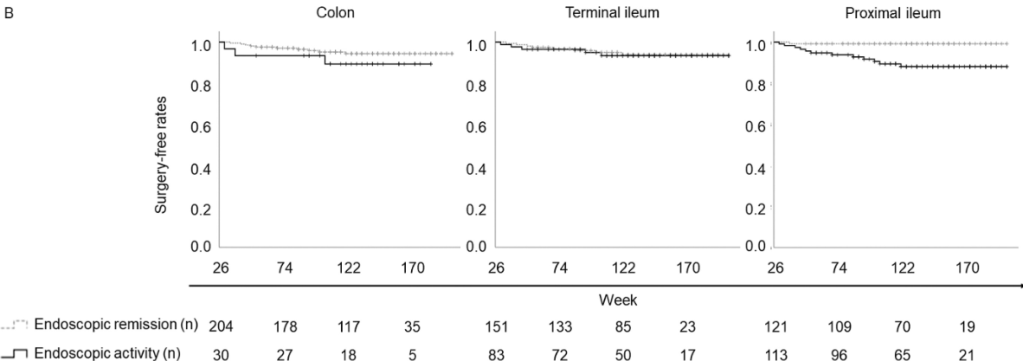
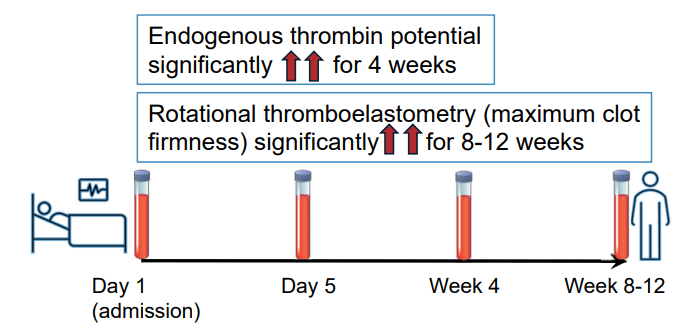
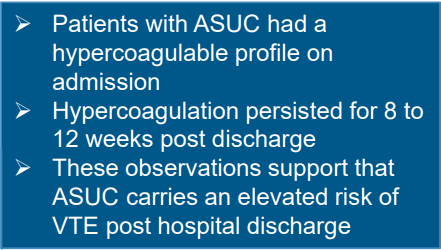
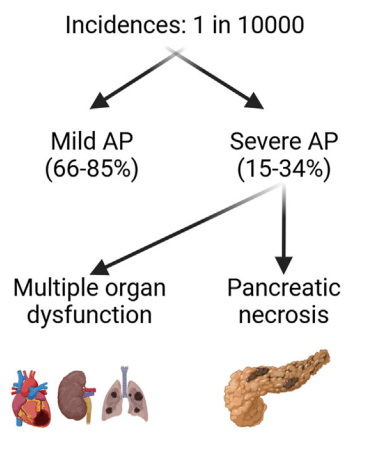
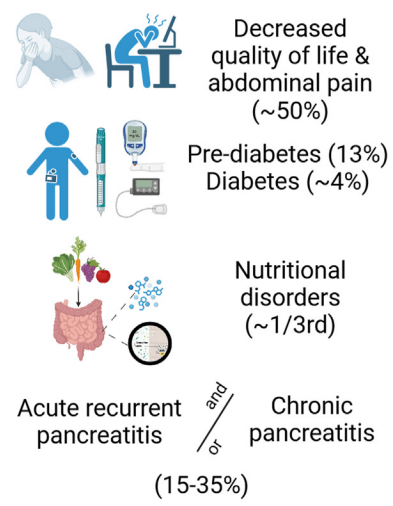
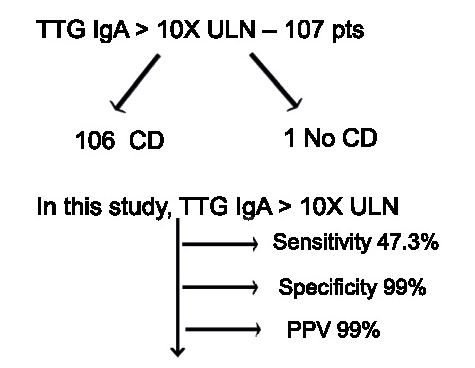
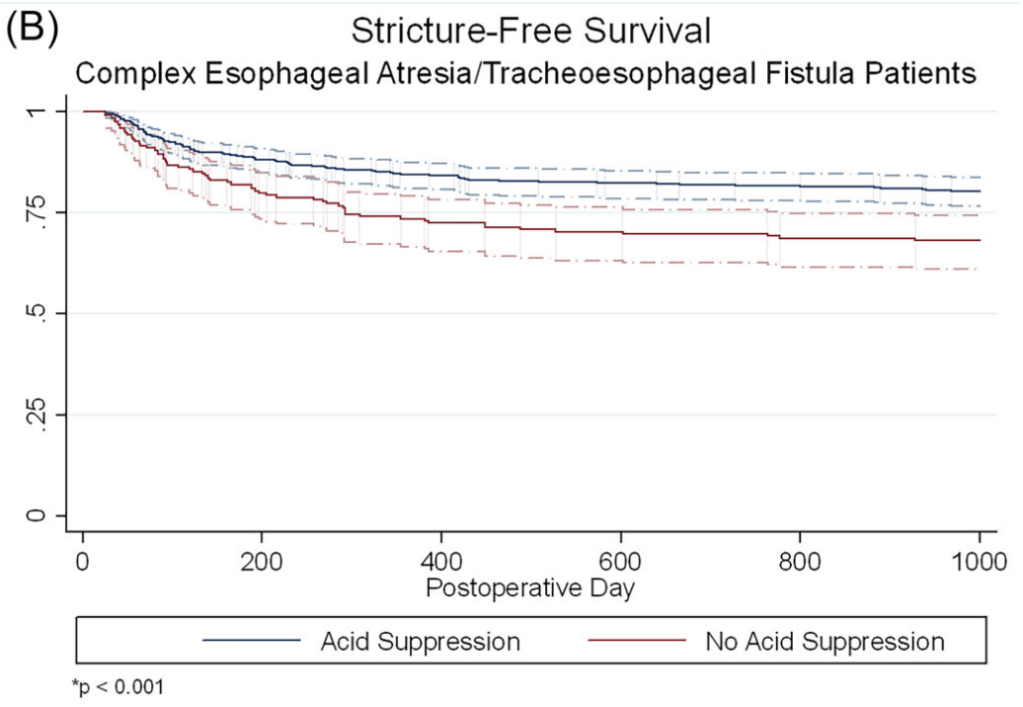
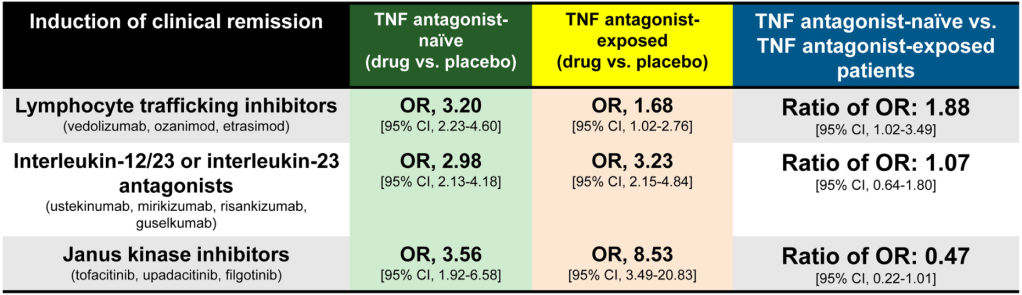
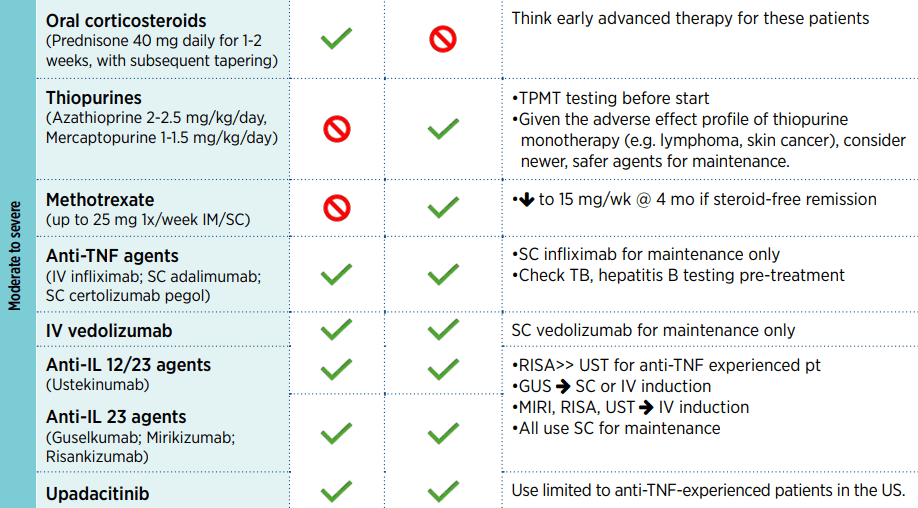
A Brooks. HCPLive 11/5/25: FDA Approves Linaclotide (Linzess) Capsules for Pediatric IBS-C
An excerpt:
The US Food and Drug Administration (FDA) has approved Ironwood Pharmaceuticals’ linaclotide (Linzess) capsules for pediatric patients ≥ 7 years of age with irritable bowel syndrome with constipation (IBS-C), making it the first treatment approved for IBS-C in this patient population.1…
The approval for pediatric IBS-C was supported by extrapolation of efficacy from adequate and well-controlled studies in adults and a 12-week double-blind, randomized, parallel-group trial in pediatric patients 7-17 years of age who met modified Rome III criteria for child/adolescent IBS-C. The recommended dosage for this indication is 145 mcg orally once daily.1…
In 2023, the FDA approved linaclotide for the treatment of pediatric patients aged 6-17 years with functional constipation at a recommended dosage of 72 mcg orally once daily.
Reference: US Food and Drug Administration. FDA approves 1st drug for children 7 years and older with irritable bowel syndrome with constipation. November 5, 2025. Accessed November 5, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-1st-drug-children-7-years-and-older-irritable-bowel-syndrome-constipation
Also, NBC news (11/08/25): ByHeart baby formula recalled amid 10-state outbreak of infant botulism The U.S. Food and Drug Administration said the outbreak includes 13 hospitalizations since August of children who consumed ByHeart Whole Nutrition Infant Formula. No deaths have been reported.
The recall includes two lots of the powdered formula with Dec. 1 “use by” dates, the FDA said in a statement Saturday. The lot numbers are 206VABP/251261P2 and 206VABP/251131P2…The FDA says ByHeart makes up less than 1% of the baby formula sold in the U.S.