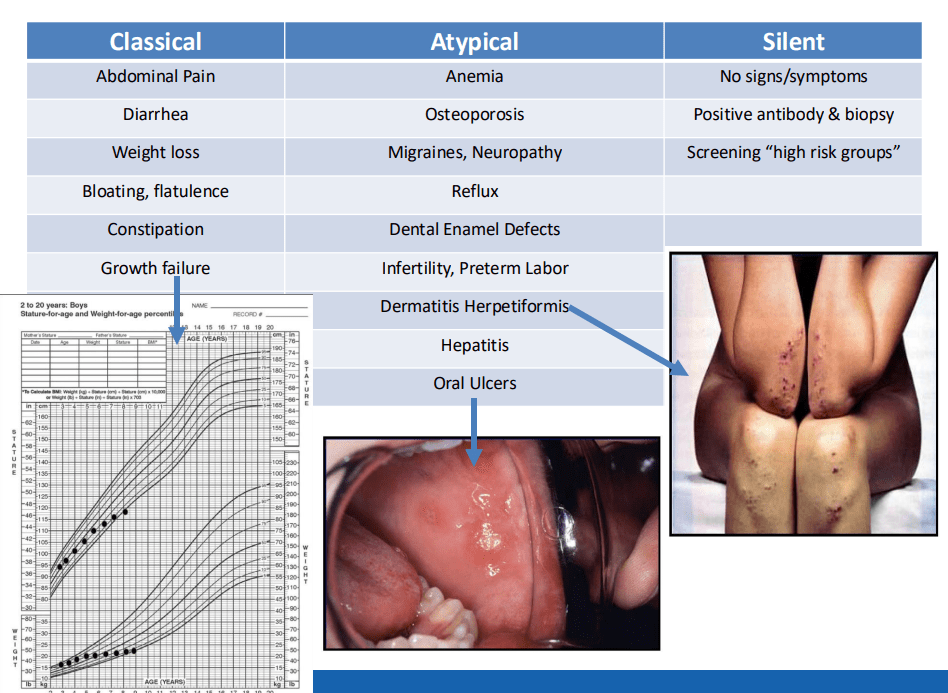
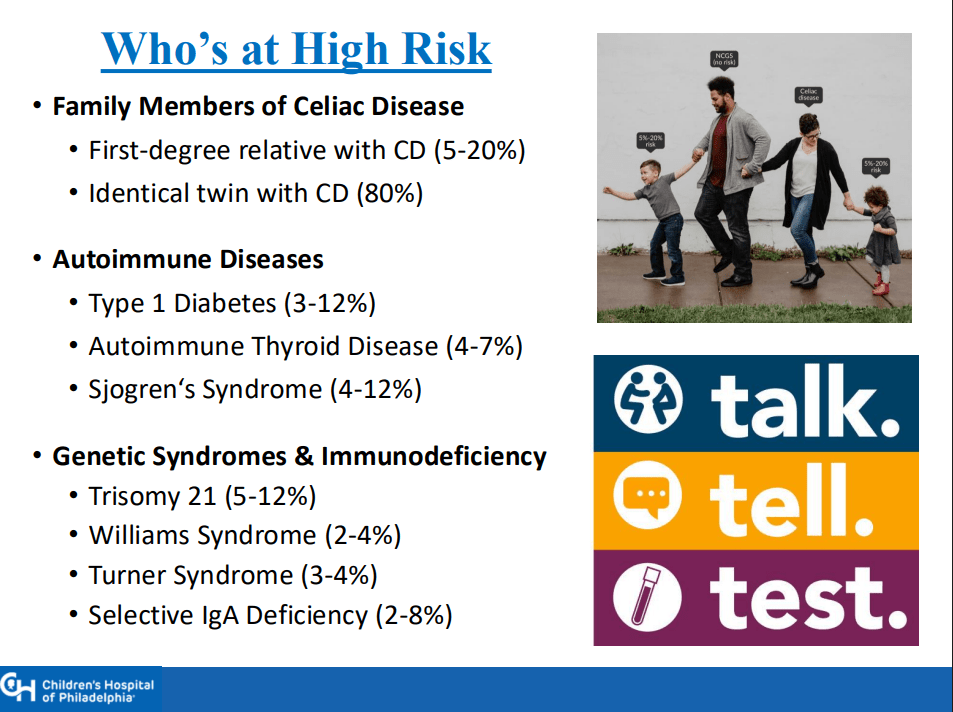
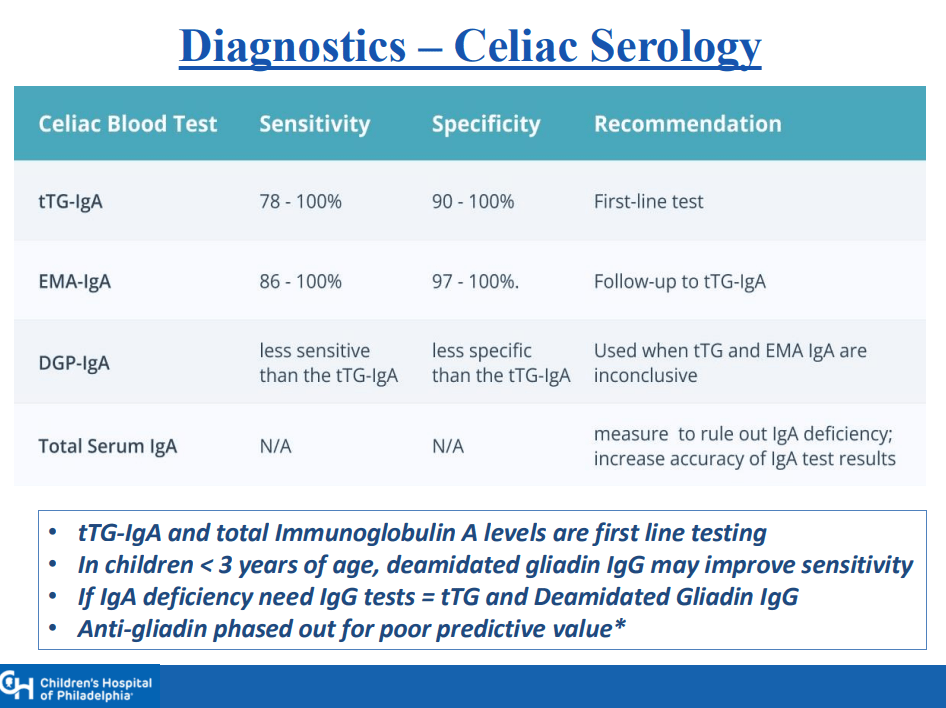
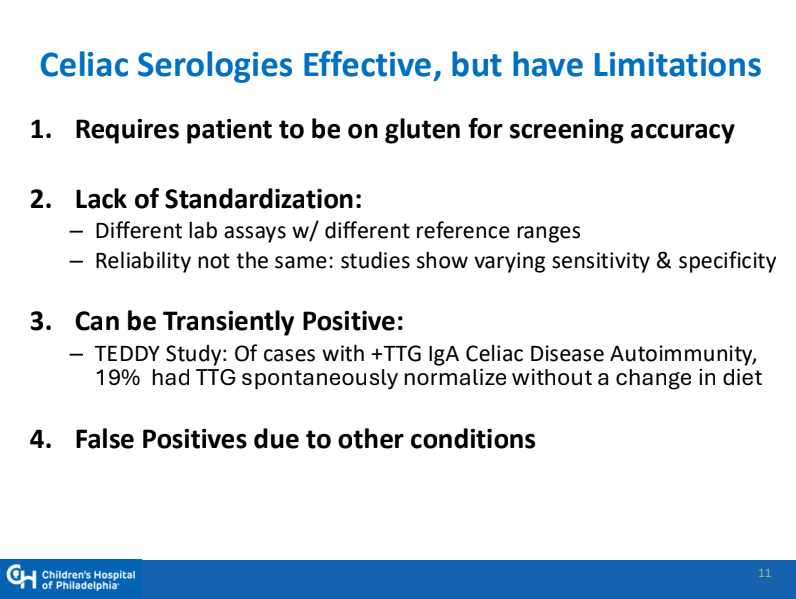
CA Ostertag-Hill et al. J Pediatr 2025;281:114523. The Natural History of Congenital Hepatic Hemangiomas
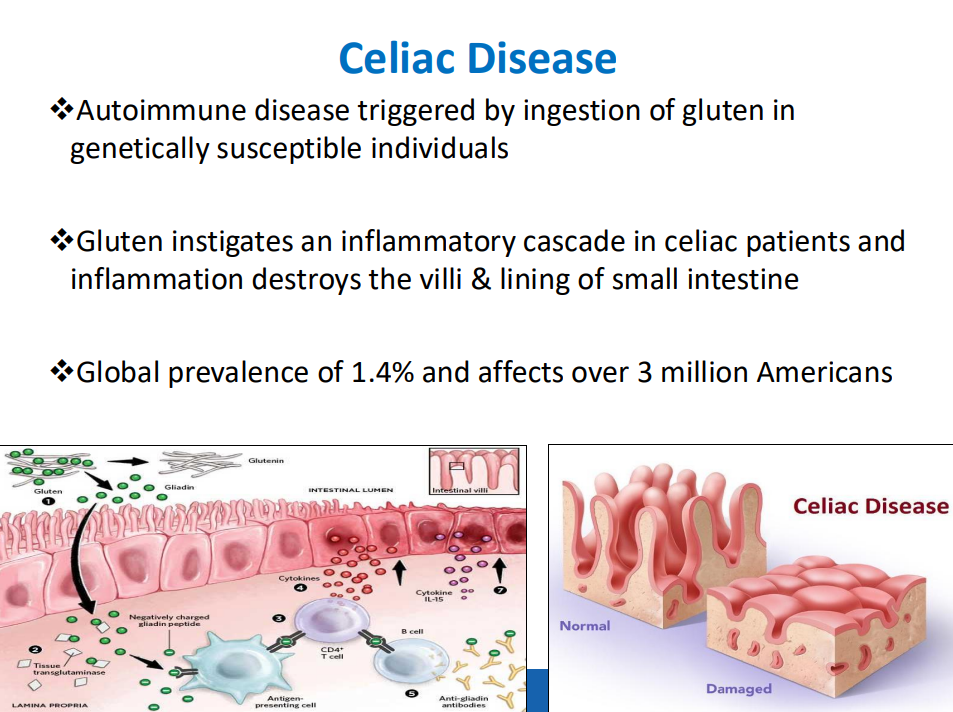
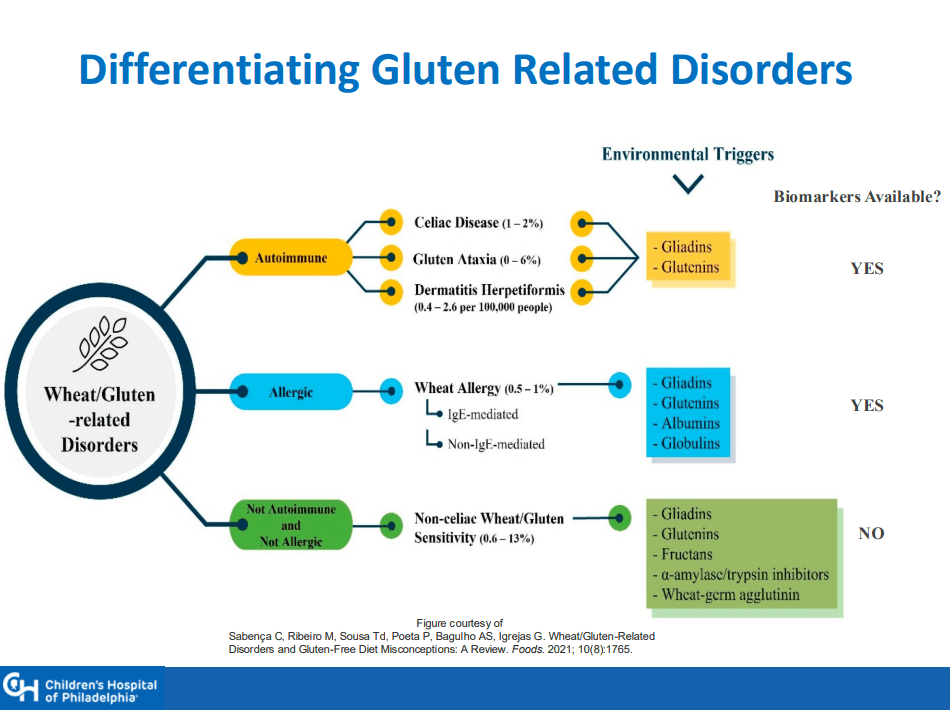
Background: “Hemangiomas remain the most common tumor of the liver in newborns and infants…A subtype classification system for hepatic hemangioma (HH) was first proposed based on our Liver Hemangioma Registry in 2007, delineating focal, multifocal, and diffuse HH…Focal hepatic lesions correspond to congenital hemangiomas whereas multifocal and diffuse lesions represent infantile hemangiomas.”
Methods: This was a retrospective review of 96 infants over an 18 year period (2004-2022). Patients with infantile HH were excluded.
Key Findings:
- 32% were diagnosed prenatally, 23% developed heart failure, and 23% developed respiratory failure
- There was a balanced sex distribution (50% for each gender)
- Common clinical features included transient anemia (n = 23/48, 48%) and thrombocytopenia (n = 30/53, 57%).
- On average, patients demonstrated 43% residual HH volume at 12 months and 16% residual volume at 24 months
- No difference in time to 50% HH volume reduction between patients with and without medical therapy was observed
- Larger hemangioma volumes were associated with an increased risk of anemia (P = .005) and thrombocytopenia (P < .001)
- There was not a significant association between HH volume and congestive heart failure (CHF) or cardiomegaly. For example, the HH volume was 824 mL vs 579 mL (P=0.689) in those with and without CHF, respectively
Discussion Points:
- “Congenital HH is present at birth and typically does not undergo postnatal growth”
- “Congenital HH occurs equally in males and females and is immunonegative for GLUT-1. This stands in contrast to infantile hepatic and cutaneous hemangioma, which exhibits an early proliferative phase followed by gradual involution”
- “HH size at diagnosis was associated with respiratory failure but not with the development of cardiomegaly or CHF, suggesting intralesional shunting may not be related to absolute tumor volume. Hence, all HH regardless of size should be assessed for shunting by doppler US”
- “We advocate consideration of cross-sectional imaging and/or biopsy if patients with presumed congenital HH do not follow the expected clinical behavior of early signs of involution”
- “Although corticosteroids and propranolol have proven benefit for infantile HH, we
demonstrate that there was no significant difference in the rate of congenital HH involution between patients who had received medical therapy and those that did not” - “Medical therapy does have a role in the medical management of high-output heart failure occurring secondary to shunting. If refractory to pharmacologic therapy, these patients
should undergo embolization of symptomatic intrahepatic shunts”

monitoring period and there is concern for intralesional bleeding. Repeat AFP as necessary to rule out hepatoblastoma. TFTs indicated if unclear whether congenital HH or infantile HH
.
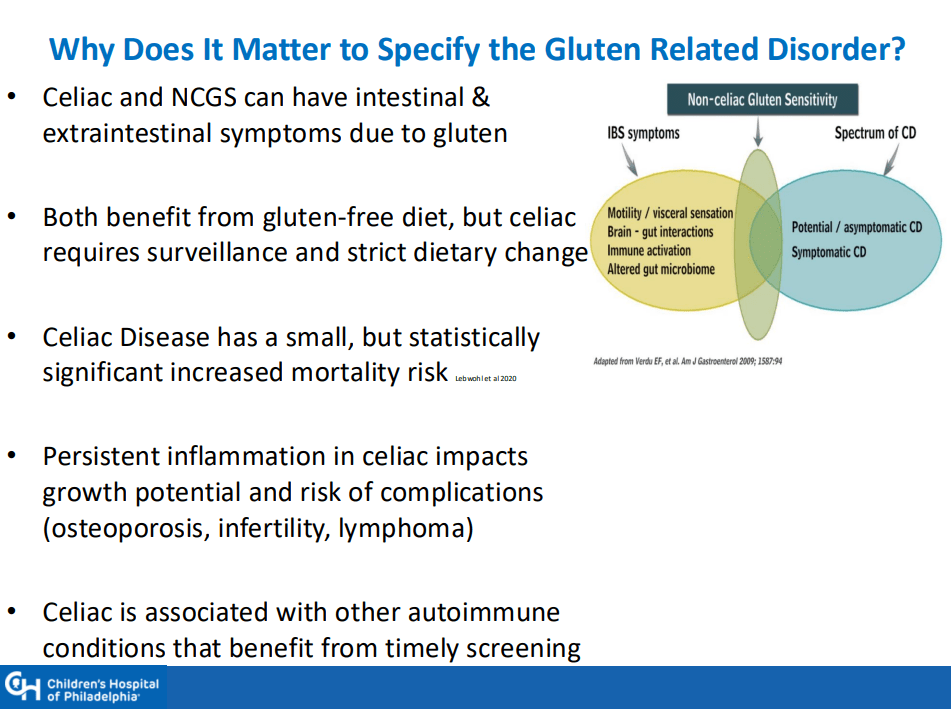
My take: This is a very useful study. It is important to distinguish congenital HH from infantile HH. Even in those with congenital HH, “a subset of patients develop life-threatening complications including respiratory failure and CHF that warrant directed medical management.”
Related blog posts:
- Incidental Liver Lesions -What to Do
- Liver Masses -Helpful Reference
- Liver Updates: Statin Protection from HCC, PSVD -new name, novel finding and Hypothyroidism with Hepatic Hemangiomas
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.