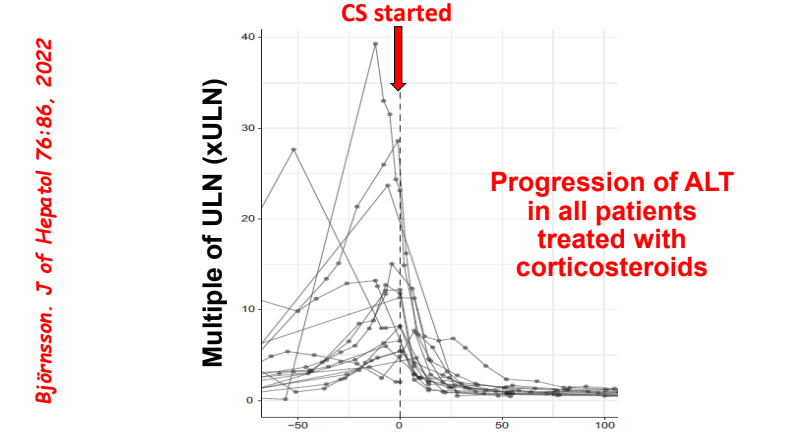
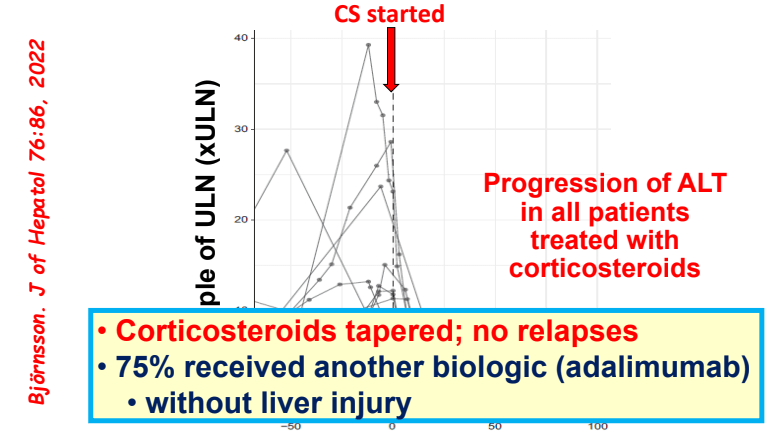
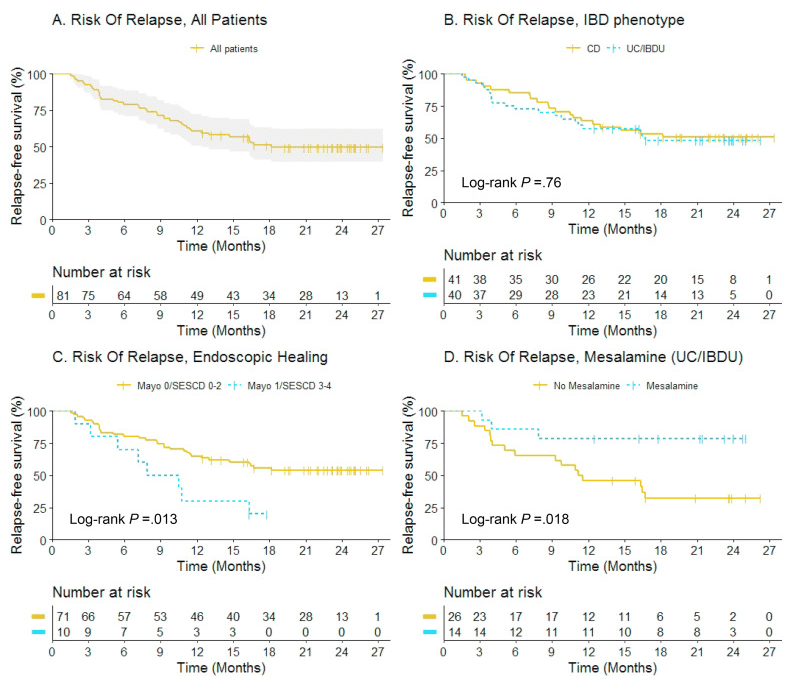
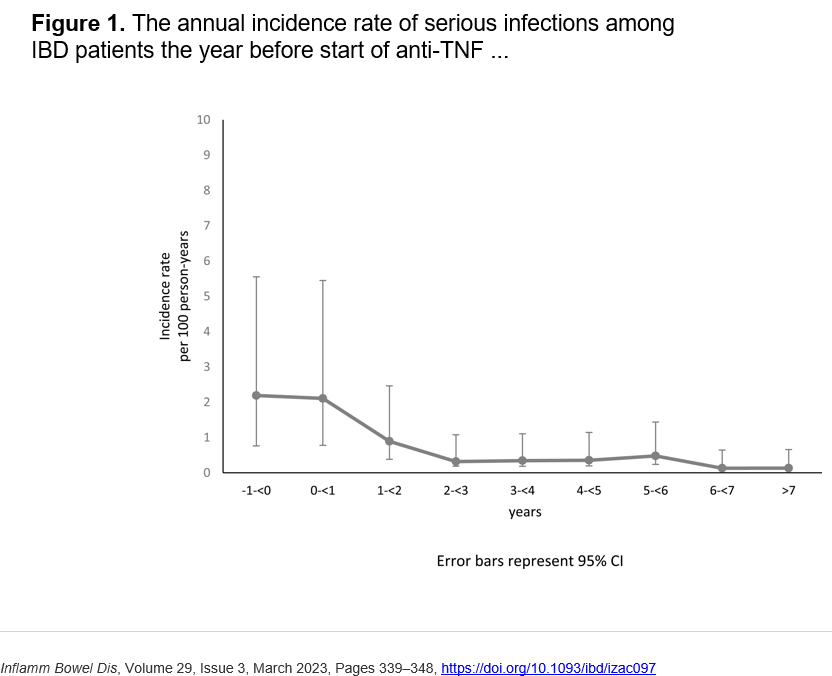
J McCurdy et al. AP&T 2025; https://doi.org/10.1111/apt.70081. The Impact of Setons on Perianal Fistula Outcomes in Patients With Crohn’s Disease Treated With Anti-TNF Therapy: A Multicentre Study
This study included 221 patients — 81 with setons and 140 without setons. Patients were treated with their first anti-TNF therapy for perianal fistulizing Crohn’s disease (PFCD) after undergoing a pelvic MRI between 2005 and 2022 from 6 North American centers. Our primary outcome was major adverse fistula outcome (MAFO), a composite of repeat local surgical intervention, hospitalization, or fecal diversion for PFCD.
Key findings:
- Patients with setons had similar rates of MAFO (HR 1.23; 95% CI, 0.68–2.21) and fistula remission at 6 months (OR, 0.81; 95% CI, 0.41–1.59) and 12 months (OR, 0.63; 95% CI, 0.31–1.27) compared to patients without setons
- In patients with abscesses, there were lower rates of MAFO (HR, 0.49; 95% CI, 0.19–1.25) but not statistically significant in patients with setons
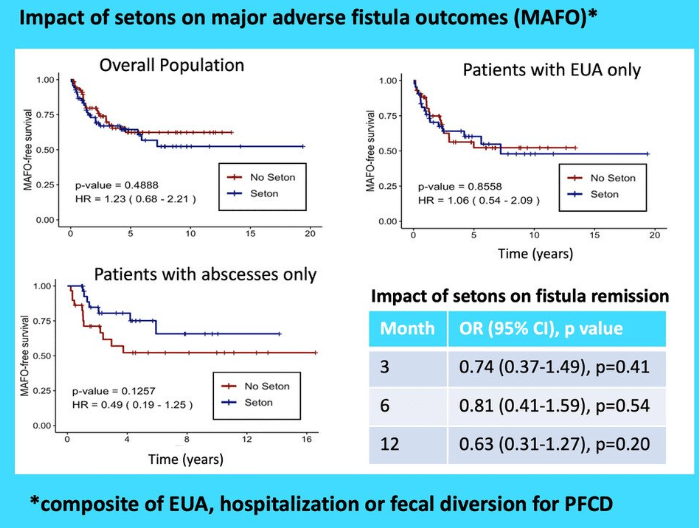
My take: This study indicates that seton placement may not be needed in patients who are starting anti-TNF therapy with fistulizing disease, especially if there is not an abscess present.
Related blog posts:
- CCFA 2023 (Atlanta) Part 5
- Silent Anal Fistulas –Sounds Bad, Is It?
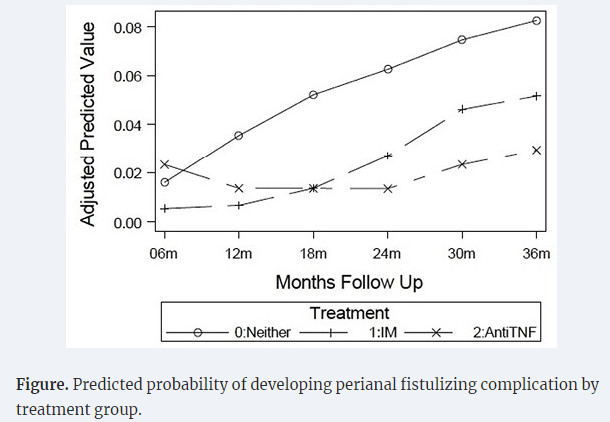
- Early Treatment Can Prevent Fistulas in Pediatric Crohn’s Disease (2024)
- Toronto Consensus for Perianal Fistulizing Crohn’s Disease (2019). “If complicated fistulizing disease, then surgical intervention may be needed prior to institution of anti-TNF therapy.”
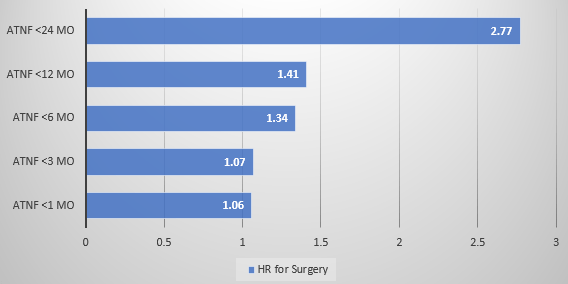
- Early Treatment with Anti-TNF Agents and Development of Perianal Fistulas
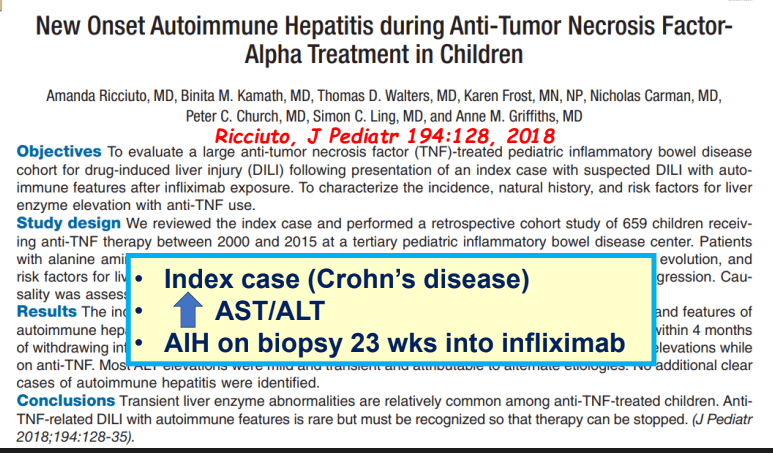
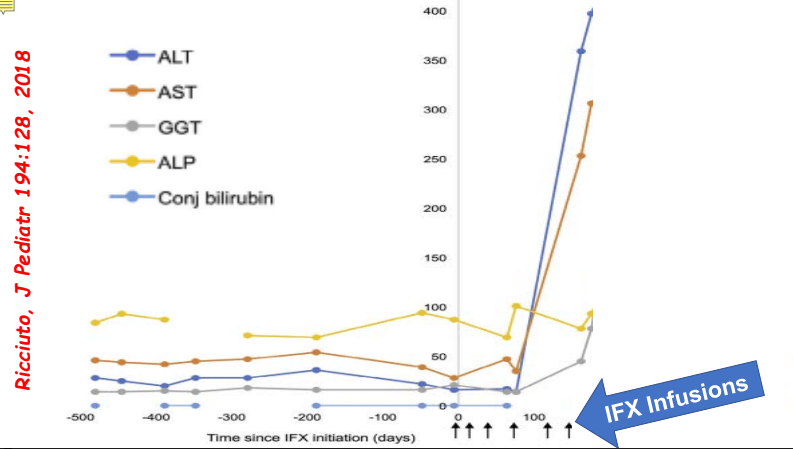
- Impact of Adalimumab Levels on Fistula Healing in Crohn’s Disease
- ENTERPRISE Study: Vedolizumab for Perianal Fistulizing Crohn’s Disease
- IBD Update -December 2020
- ADMIRE Study: Use of Stem Cell Therapy for Complex Perianal Fistulas in Crohn’s Disease
- Pediatric Consensus Statement: Perianal Crohn Disease (2013)
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.