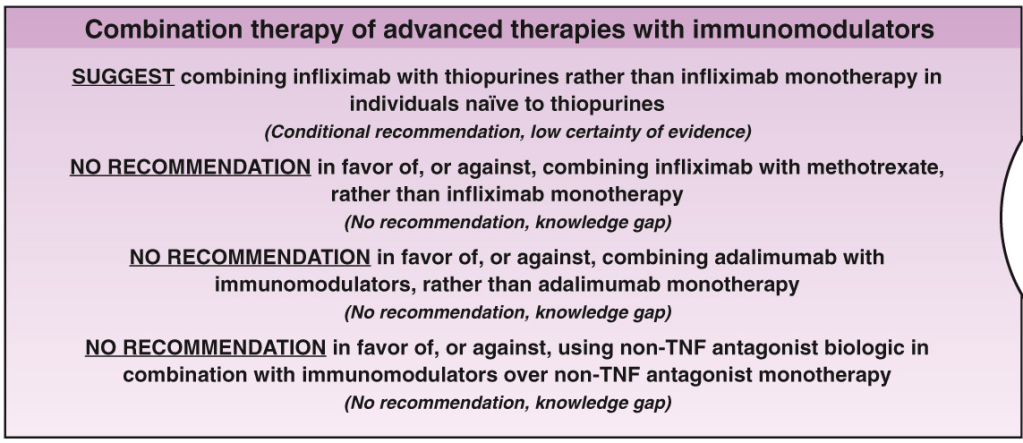
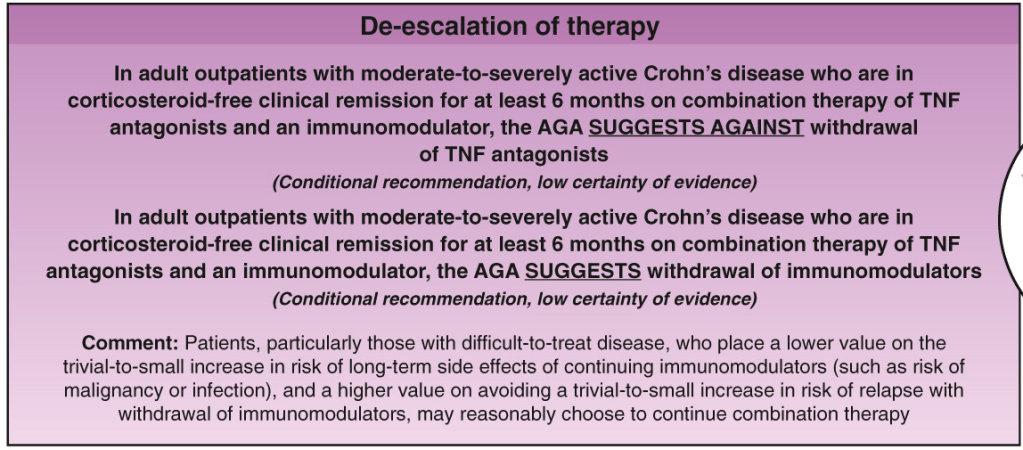
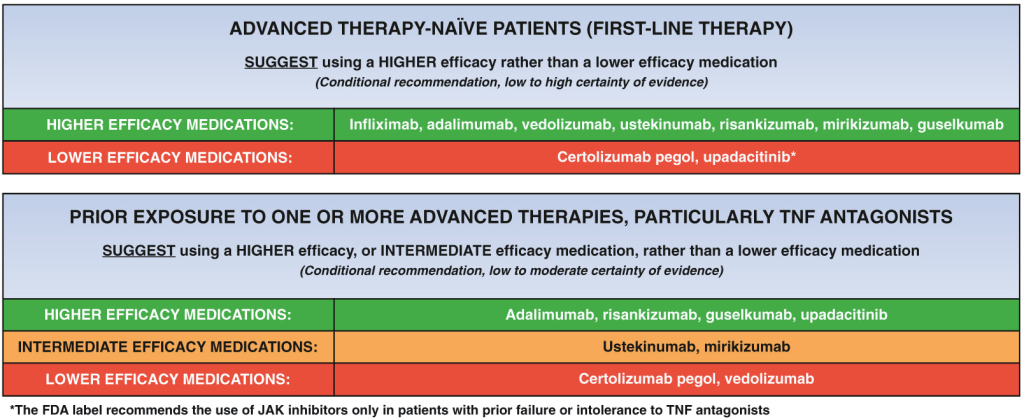
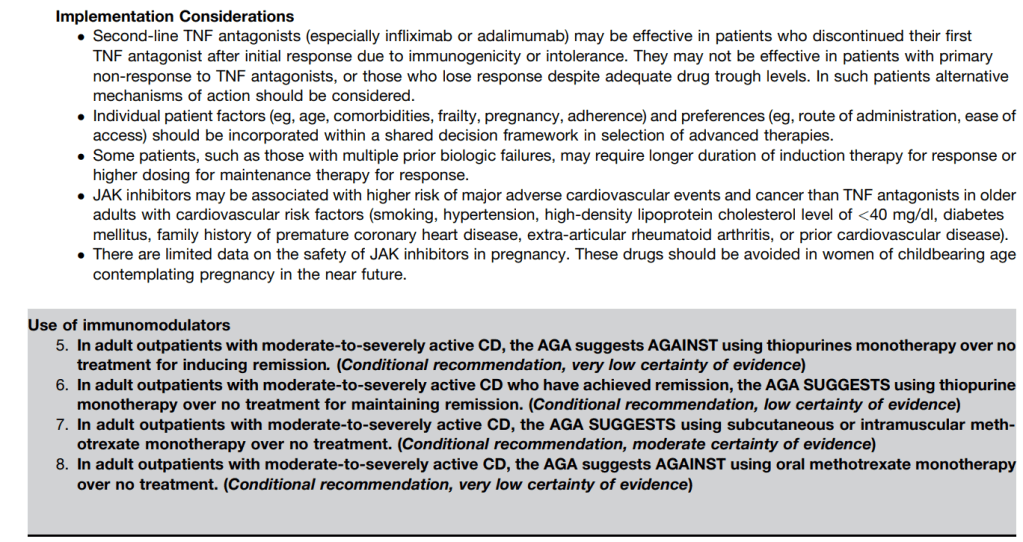
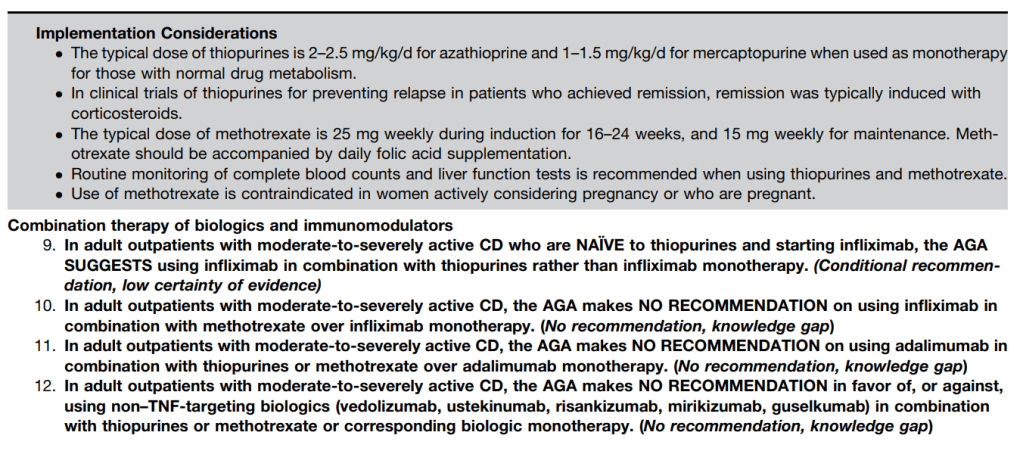
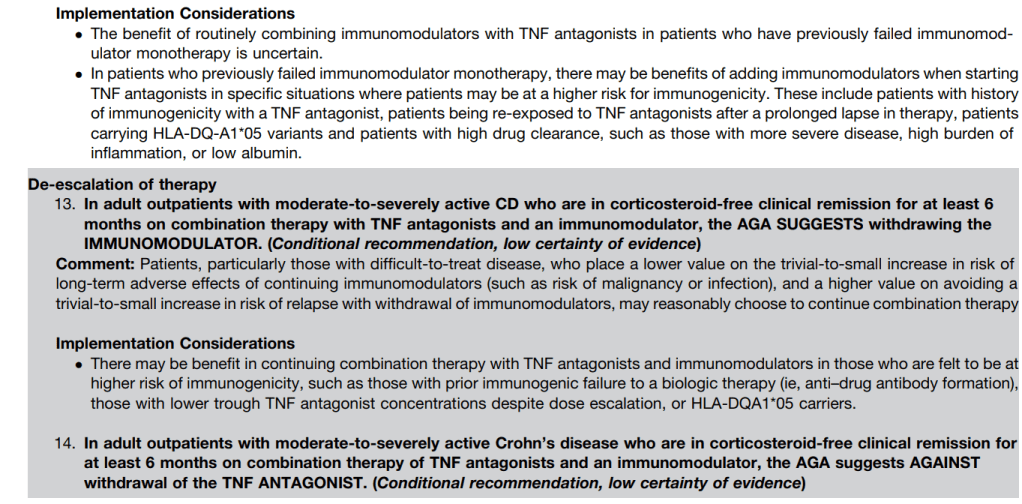
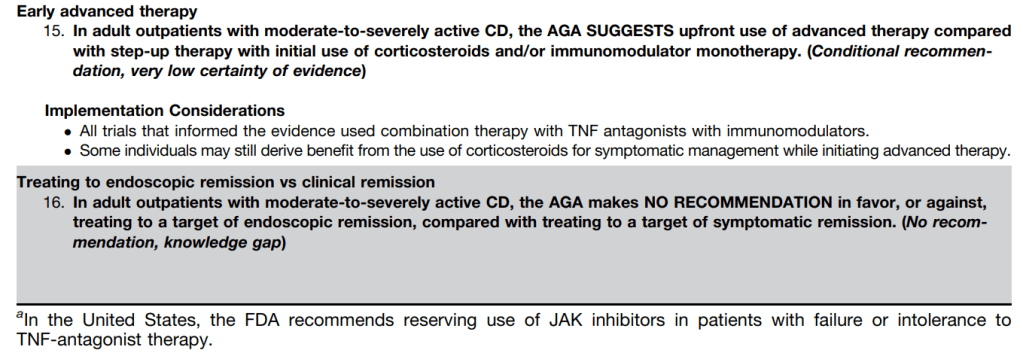
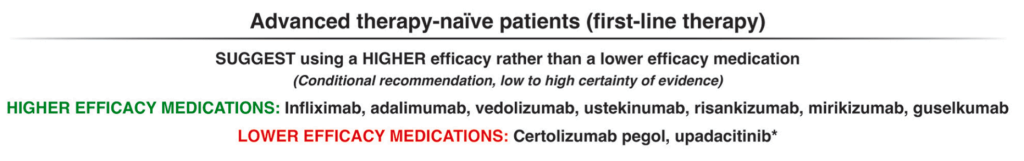
LMA Van Lierop et al. Gastroenterol 2026; 170: 404-407. Open Access! Long-Term Outcomes of Increased Versus Conventional Adalimumab Dose Interval for Patients With Crohn’s Disease in Stable Remission: 3-Year Follow-Up of the Randomized Controlled LADI Trial
Methods: “The LADI trial enrolled adults with luminal CD in corticosteroid-free clinical (CFCR) and biochemical remission, on adalimumab, 40 mg every 2 weeks. After randomization in a 2:1 ratio, the intervention group started on a 3-week interval and increased to 4 weeks, if in clinical and biochemical remission at week 24. The control group remained on adalimumab biweekly…The primary end point in this long-term follow-up (LTFU) study was the proportion of patients in CFCR (Harvey Bradshaw Index [HBI] <5 or remission per Physician Global Assessment [PGA] without systemic corticosteroids) without complications at year 3, on the assigned adalimumab interval.”
Key findings:
- The proportion of patients achieving the primary end point was 34 of 95 (35.8%, intervention) vs 41 of 48 (85.4%, control; P < 0.001).
- At year 3, 39 of 95 (41.1%) in the intervention group remained on the randomized or further de-escalated adalimumab regimen
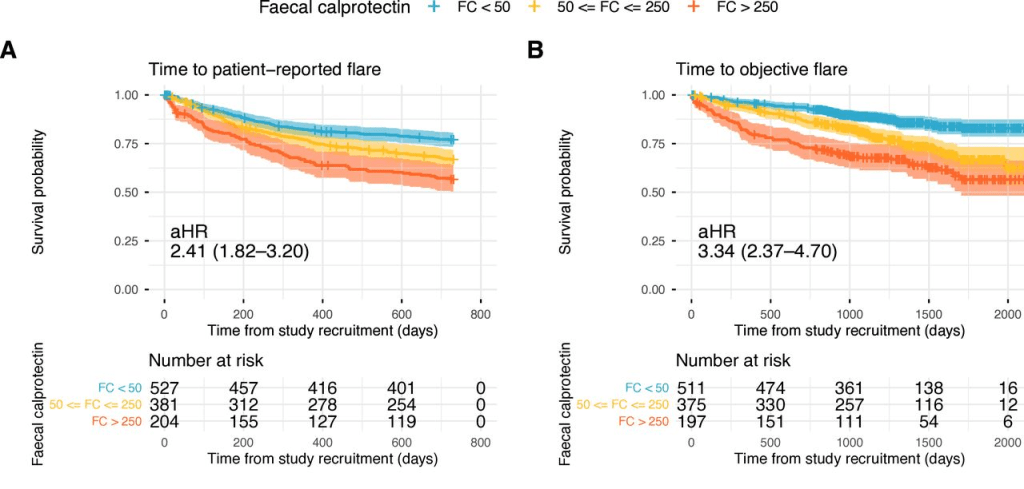
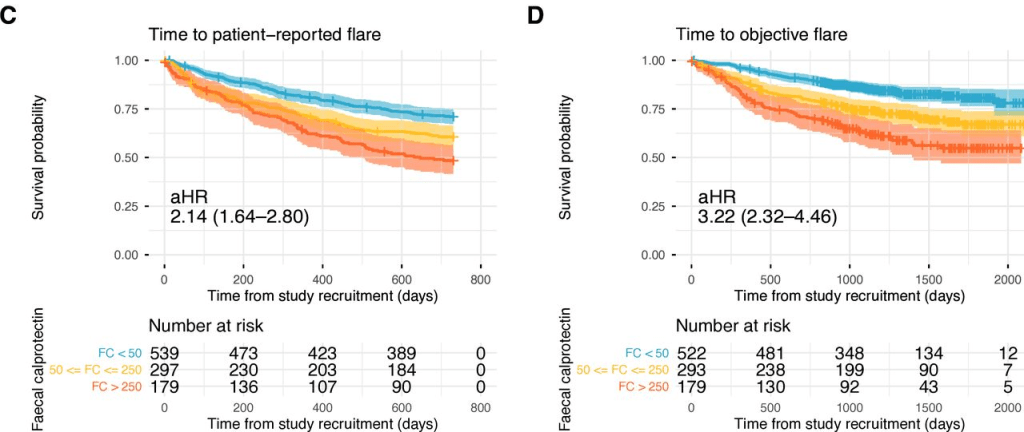
- Kaplan-Meier analyses of secondary end points showed the following probabilities at year 3 (intervention vs control) (Figure 1): remaining on the assigned adalimumab dose, 41.4% vs 91.4% (P < .0001); remaining on adalimumab, 83.7% vs 95.8% (P = .026); corticosteroid-free survival, 87.4% vs 95.7% (P = .062); and complication-free survival, 83.2% vs 97.9% (P = .015)
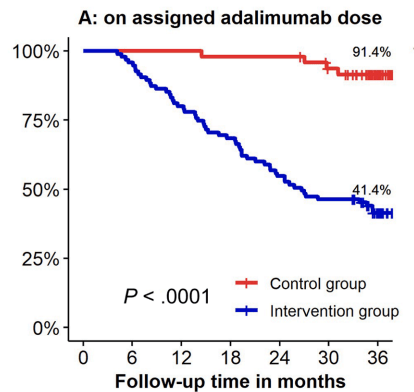
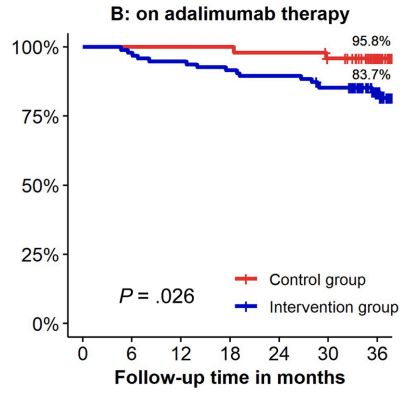
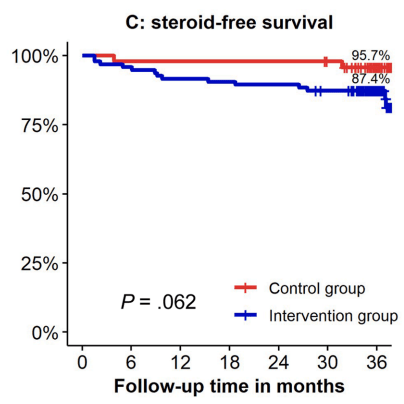
My take: About 60% of patients were unable to de-escalate their adalimumab dosing interval. Suboptimal dosing increased the risk of complications and having adalimumab therapy become ineffective.
Related blog posts:
- Better Levels –>Better Outcomes with Adalimumab
- High Relapse Rates with Anti-TNF Withdrawal Even with Endoscopic Remission
- High Risk of Relapse in Younger Patients after anti-TNF Therapy Withdrawal
- Withdrawing Therapy Leads To Relapse, Even if in Deep …
- Proactive Drug Monitoring for Crohn’s Disease in Pediatrics
- Combination Therapy Study Points to Central Role of Adequate Drug Levels
- NASPGHAN Pediatric Position Paper for Therapeutic Drug Monitoring
- “Do Not Stop Anti-TNF Medications in Children with IBD When They Are Working”