Congratulations to Dr. Benjamin Gold who is one of the honored heros at this year’s CCFA Take Steps. Also, congratulations to Clara Cann and Lauren Leonard who are being recognized as well.
Link: Register for Take Steps 2025 (4/26/25)


Congratulations to Dr. Benjamin Gold who is one of the honored heros at this year’s CCFA Take Steps. Also, congratulations to Clara Cann and Lauren Leonard who are being recognized as well.
Link: Register for Take Steps 2025 (4/26/25)

John Barnard MD gave a great talk today as part of the yearly Donald Schaffner lecture. This lecture also honored Larry Saripkin (see blog post: Thank You Larry) as a master clinician. My notes below may contain errors in transcription and in omission. Along with my notes, I have included many of his slides.
Key Points:






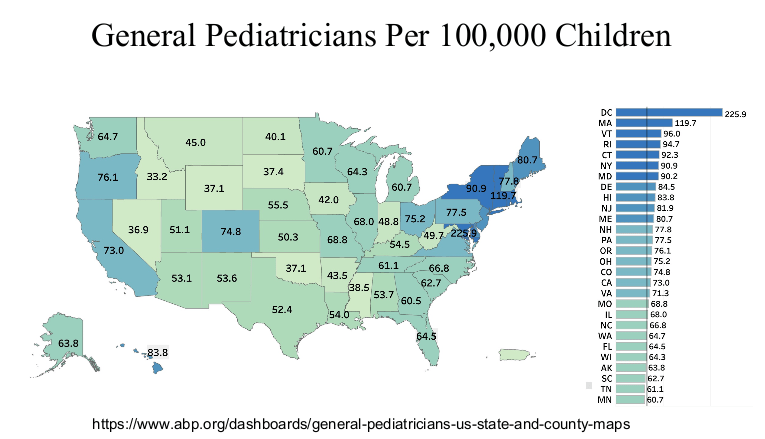






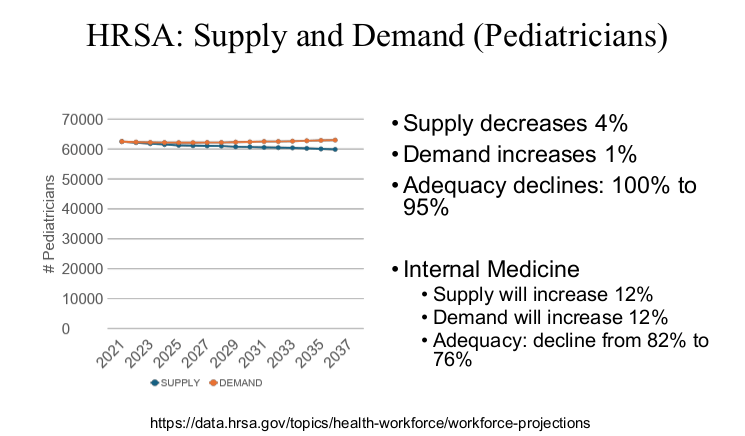

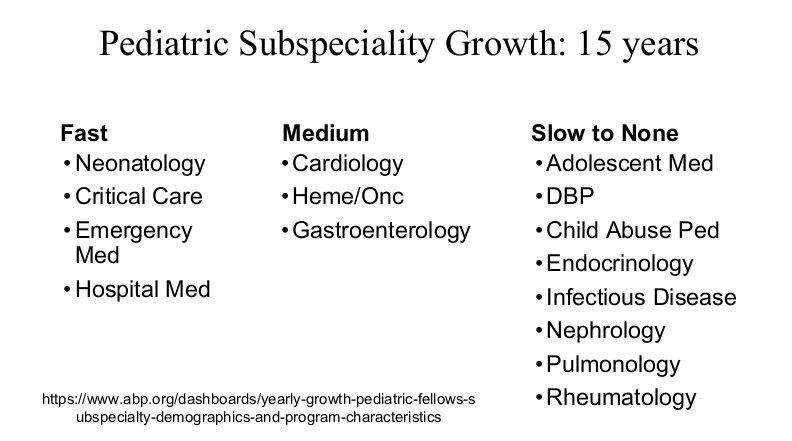


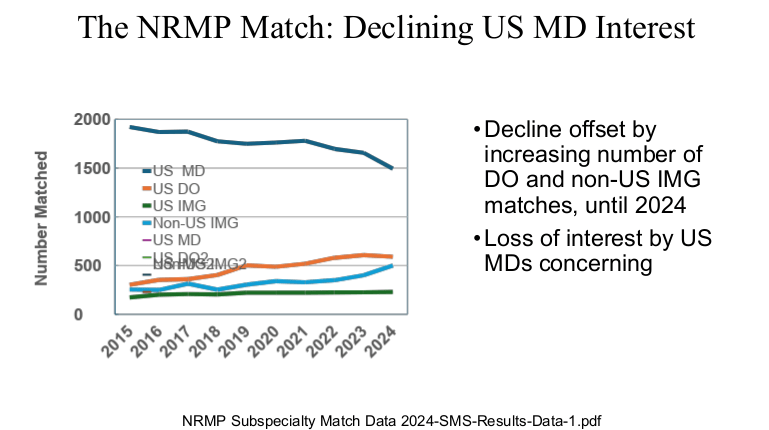

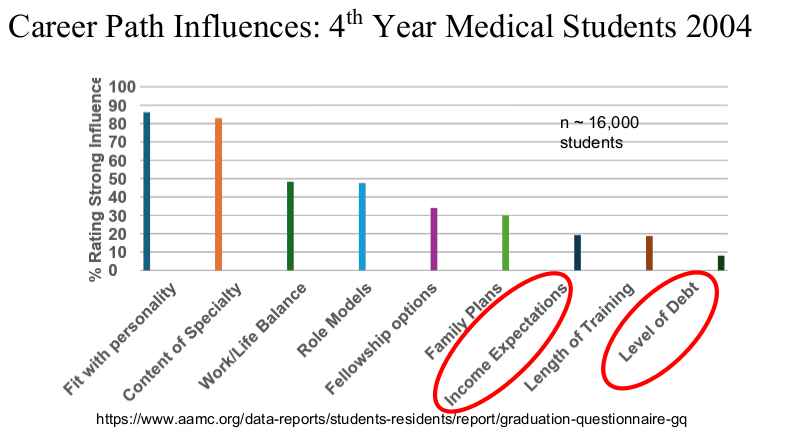
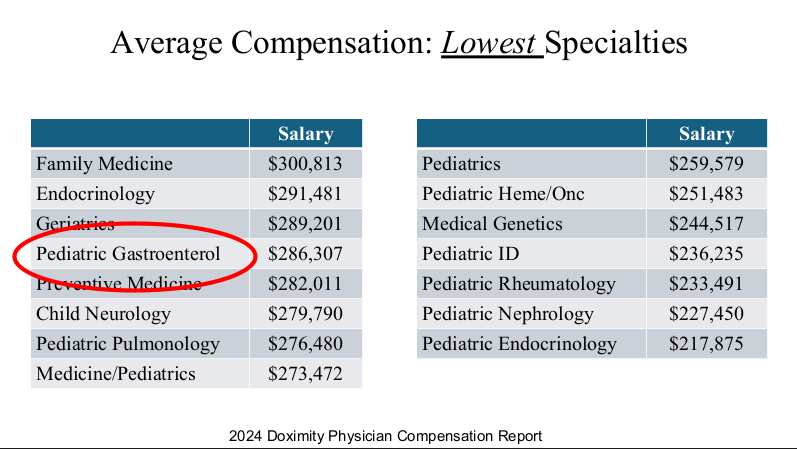
Physicians are in the top 5% of compensation, though pediatric physicians receive less
compensation than their peers. According to 2024 Doximity survey
(https://press.doximity.com/reports/doximity-physician-compensation-report-2023.pdf)
average physician salary exceeds $350,000 in most metro areas.









My take: Dr. Barnard noted that “medicine has never been more exciting than it is today.” Yet, the decreased interest of medical students for a career in pediatrics/pediatric subspecialties needs to be addressed.
Dr. Barnard modified the material and presented the William Balistreri lecture at this year’s NASPGHAN meeting. Here are some additional slides from this talk which focused more on Pediatric Gastroenterology:



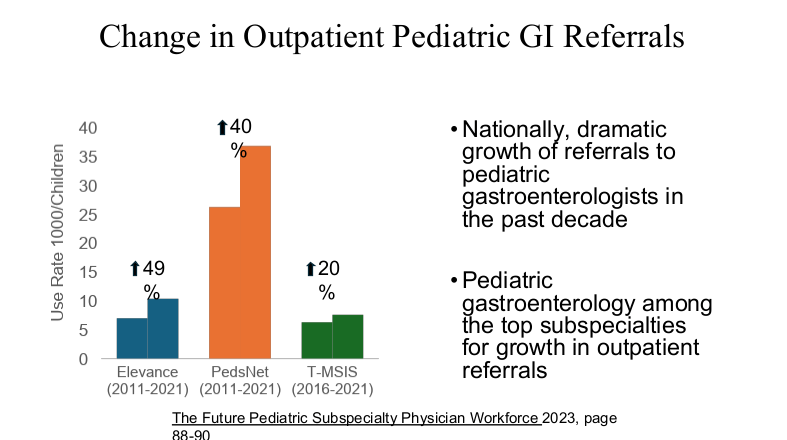
Related blog post: “Why It’s So Hard to Find a Pediatrician These Days”
A recent study (below) reminded me of a joke. First the joke (better with the visual effect):
A guy goes to his doctor. The patient says, “Doctor when I touch here on my shoulder (with index finger) it hurts, when I touch here on my leg (with index finger) it hurts, and when I touch here on my stomach (with index finger) it hurts.”
The doctor says: “Your finger is broken.”
BP Chumpitazi et al. J Pediatr 2021; 236; 131-136. Multisite Pain Is Highly Prevalent in Children with Functional Abdominal Pain Disorders and Is Associated with Increased Morbidity
In this cross-sectional study of 7-17 year olds (n=406) with Rome III functional abdominal pain disorder (FAPD), the authors examined the frequency of pain outside GI tract over a 2 week study period. Patients were recruited from both a large academic pediatric GI practice and general pediatric offices in same hospital system.
Key findings:
The authors note that due to the design of their study, they cannot establish a causal association between pain symptoms and psychosocial functioning.
My take: A lot of kids with stomach pain have multisite pain as well as anxiety and depression. This study reminds us to ask about them.
Related blog posts:


In a nationwide retrospective cohort from The Netherlands (pop. ~17 million), a recent study (RM de Voer et al. Clin Gastroenterol Hepatol 2021; 19: 1642-1651. Full Text: Clinical, Pathology, Genetic, and Molecular Features of Colorectal Tumors in Adolescents and Adults 25 Years or Younger) characterizes the clinical and genetic features of colorectal cancer (CRC) in individuals <26 years of age (aka AYA group) from 2000-2017.
Key findings:
Related blog posts:

Briefly noted: A recent survey study (N Du et al. JPGN Reports: 2021; 2: p e033. doi: 10.1097/PG9.0000000000000033. Full Text: Assessment of Community Pediatric Providers’ Approach to Children With Helicobacter pylori) found that pediatric providers had poor knowledge and/or adherence to pediatric H pylori guidelines.
Key findings:
My take: It would be interesting to compare pediatric gastroenterology provider responses to general pediatric providers. It is likely that a much higher percentage would be following established guidelines. One area of the guidelines that I think should be changed would be encouraging increased use of quadruple therapy in children, especially if resistance testing is not performed; this change would better align with adult guidelines. In adults, quadruple therapy has been associated with increased cure rates.
Related blog posts:

DR Mack et al. Gastroenterol 2019; 157: 320-48. Full Text: Canadian Association of Gastroenterology Clinical Practice Guideline for the Medical Management of Pediatric Luminal Crohn’s Disease
“When the consensus group met in October 2017, the most recent consensus guidelines for the treatment of CD in pediatric patients were those from” ESPGHAN/ECCO in 2014 with data from June 2013. Thus, the guideline attempts to provide more updated information and recommendations based on incorporating the latest studies.
The authors provide 25 consensus statements. Here are a few of interest:
In addition, the authors provide 13 statements with no recommendations -here are two of them:
Link to full article: Updated Hepatitis B Treatment Guidelines from AASLD
With regard to pediatrics:
9A. The AASLD suggests antiviral therapy in HBeAg-positive children (ages 2 to <18 years) with both elevated ALT and measurable HBV DNA levels, with the goal of achieving sustained HBeAg seroconversion.
“Most studies required ALT elevation (>1.3 times ULN) for at least 6 months with HBV DNA elevations for inclusion. Given that HBV DNA levels are typically very high during childhood (>106 IU/mL), there is no basis for a recommendation for a lower-limit value with respect to treatment. However, if a level <104 IU/mL is observed, therapy might be deferred until other causes of liver disease and spontaneous HBeAg seroconversion are excluded.”
“Duration of treatment with oral antivirals that has been studied is 1-4 years. It may be prudent to use HBeAg seroconversion as a therapeutic endpoint when oral antivirals are used, continuing treatment for an additional 12 months of consolidation, as recommended in adults. It is currently unknown whether a longer duration of consolidation would reduce rates of virological relapse.”
“Children who stop antiviral therapy should be monitored every 3 months for at least 1 year for recurrent viremia, ALT flares, and clinical decompensation.”
9B. The AASLD recommends against use of antiviral therapy in HBeAg-positive children (ages 2 to <18 years) with persistently normal ALT, regardless of HBV DNA level.
Another nice summary of current treatment recommendations: P Martin et al. Clin Gastroenterol Hepatol 2015; 13: 2071-87. Table 5 lists recommendations for treatment of HBeAg-positive.
While entecavir and tenofovir have been in use for many years in adult hepatology for hepatitis B virus (HBV) infection, a well-designed study supporting their use in pediatrics has been lacking until now. Recently, a study (M Jonas et al. Hepatology 2015; DOI: 10.1002/hep.28015) has shown that entecavir is effective for pediatric HBV
Link to full study. Randomized Controlled Trial of Entecavir Versus Placebo in Children with HBeAg-positive Chronic Hepatitis B
Here’s the abstract:
This ongoing, randomized phase III study assesses the safety and efficacy of entecavir versus placebo in nucleos(t)ide-naive children (2 to <18 years) with HBeAg-positive chronic hepatitis B (CHB). Blinded treatment was administered for a minimum of 48 weeks. After Week 48, patients with HBeAg seroconversion continued blinded treatment; those without, switched to open-label entecavir. The primary endpoint was HBeAg seroconversion and HBV DNA <50 IU/mL at Week 48. A total of 180 patients were randomized (2:1) and treated. Baseline median age was 12 years, with approximately 50% of children aged >12 to <18, and 25% each aged ≥2 to ≤6 and >6 to ≤12. Rates for the primary endpoint at Week 48 were significantly higher with entecavir than placebo (24.2% [29/120] versus 3.3% [2/60]; P=0.0008). Furthermore, higher response rates were observed with entecavir compared with placebo for the key Week 48 secondary endpoints: HBV DNA <50 IU/mL (49.2% [59/120] versus 3.3% [2/60]; P < 0.0001), alanine aminotransferase normalization (67.5% [81/120] versus 23.3% [14/60]; P < 0.0001), and HBeAg seroconversion (24.2% [29/120] versus 10.0% [6/60]; P = 0.0210). Among entecavir-randomized patients there was an increase in all efficacy endpoints between Weeks 48 and 96, including an increase from 49% to 64% in virologic suppression. The cumulative probability of emergent entecavir resistance through Years 1 and 2 of entecavir was 0.6 and 2.6%, respectively. Entecavir was well tolerated with no observed differences in adverse events or changes in growth compared with placebo. Conclusion: In childhood CHB, entecavir demonstrated superior antiviral efficacy to placebo with a favorable safety profile. These results support the use of entecavir as a therapeutic option in children and adolescents with CHB.
Related blog posts:
The authors of a recent report (JPGN 2014; 59: 409-16) acknowledge that “bowel regimens vary significantly” and “few clinical studies in pediatrics have evaluated the use of various bowel preparation regimens.” Furthermore, “pediatric studies did not have a common efficacy measure.”
Nevertheless, they provide a “NASPGHAN best practices cleanout regimens.” According to Table 7:
My personal opinion is that Table 7 could drop the words “best practices” since the report states “alternative dosing regimens may be entirely reasonable” and the data are quite limited.
With regard to split dosing preparations which are now recommended in adults, their role in pediatrics is a “potential area for future research.” For adults, the U.S. Multi-Society Task Force Consensus Statement on Adequate Bowel Cleansing for Colonoscopy (Johnson DA et al. Optimizing Adequacy of Bowel Cleansing for Colonoscopy: Recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014; 147(4):903-924) recommends:
Take-home message: This NASPGHAN report summarizes the literature and provides recommendations for effective bowel preparations.
Related blog posts: