NY Times 8/19/25: Are Marathons and Extreme Running Linked to Colon Cancer?
An excerpt:
A small, preliminary study found that marathoners were much more likely to have precancerous growths. Experts aren’t sure why…
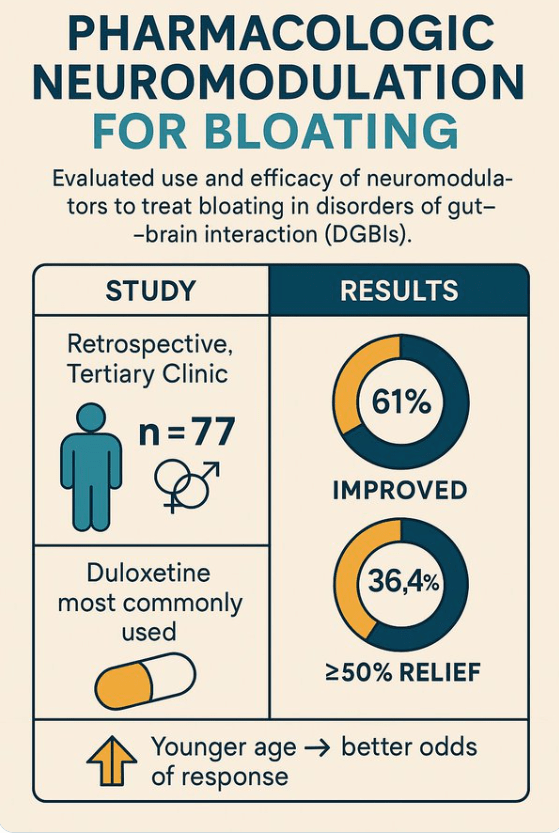
Dr. Cannon, an oncologist with Inova Schar Cancer in Fairfax, Va., launched a study, recruiting 100 marathon and ultramarathon runners aged 35 to 50 to undergo a colonoscopy.
The results were staggering. Almost half the participants had polyps, and 15 percent had advanced adenomas likely to become cancerous. The rate of advanced adenomas was much higher than that seen among adults in their late 40s in the general population, which ranges from 4.5 percent to 6 percent, according to recent studies.
The research was presented at an American Society of Clinical Oncology conference but has not yet been published in a medical journal…
Dr. David Rubin, chief of gastroenterology and director of the Inflammatory Bowel Disease Center at the University of Chicago, said the study was important but limited. It lacked a control arm consisting of similar young adults who were not long-distance runners, he noted, and the family histories of colon cancer among the marathoners were not entirely known…
Runners often develop gastrointestinal symptoms that they dismiss as benign — so-called runner’s trots, for example. The symptoms can be caused by ischemic colitis, a condition that develops when blood flow to the colon is temporarily reduced as it is redirected to muscles in other parts of body (like a runner’s legs).
My take: While this is a small study, it indicates that extreme runners could have an increased risk of colonic polyps and cancer. If there are symptoms (especially rectal bleeding and weight loss), a low threshold for further evaluation is needed.
Related blog posts:
- How Gut Bacteria Might Increase Colon Cancer Risk in Young Adults
- Colon Cancer at Younger Ages
- Colorectal Cancer in Patients Up to Age to 25 Years
- Sugary Diet and Colonic Adenomas