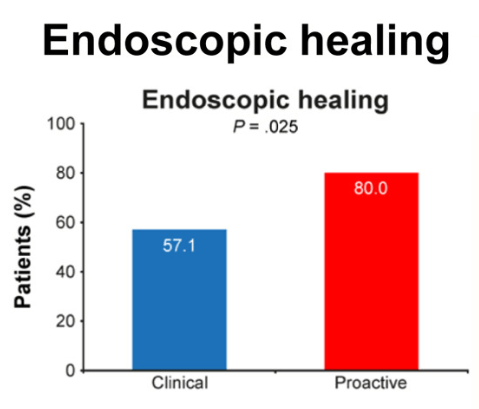
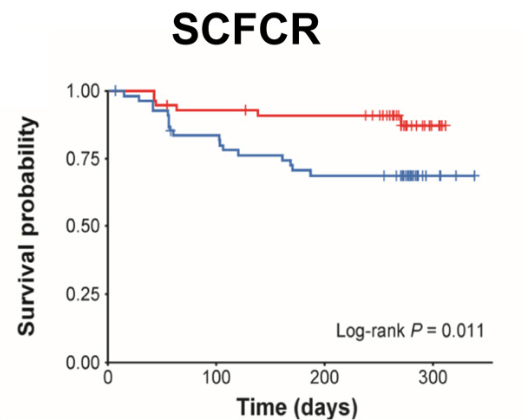
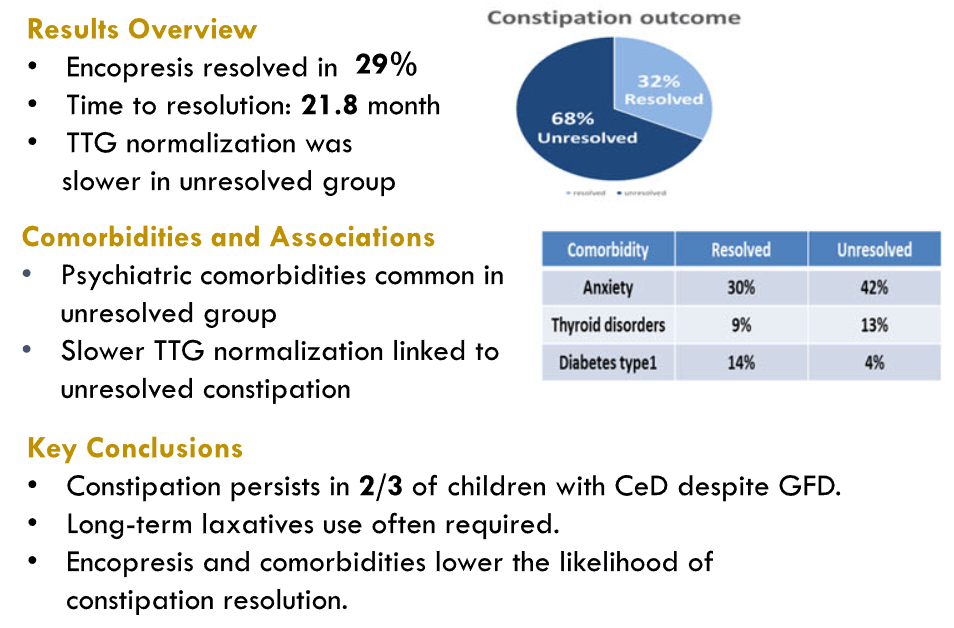
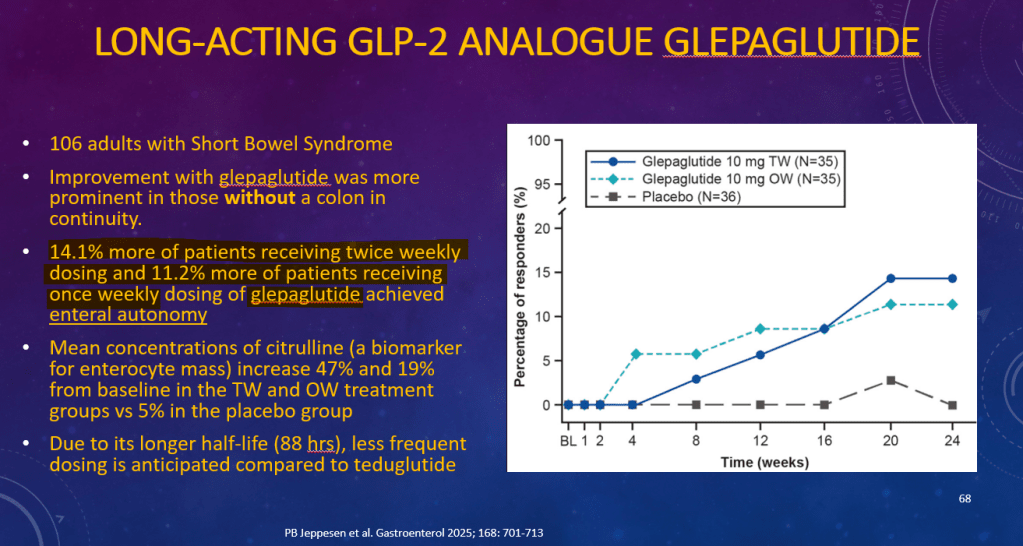
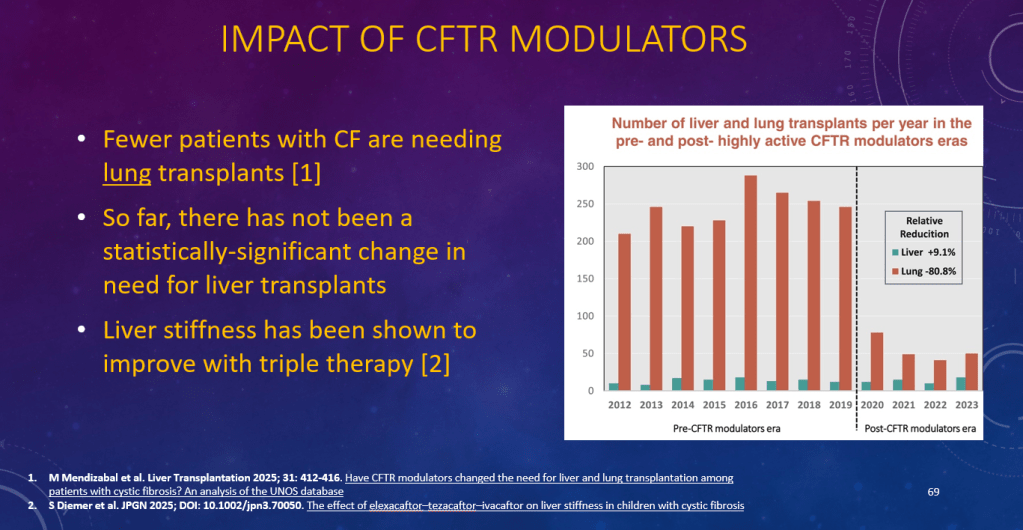
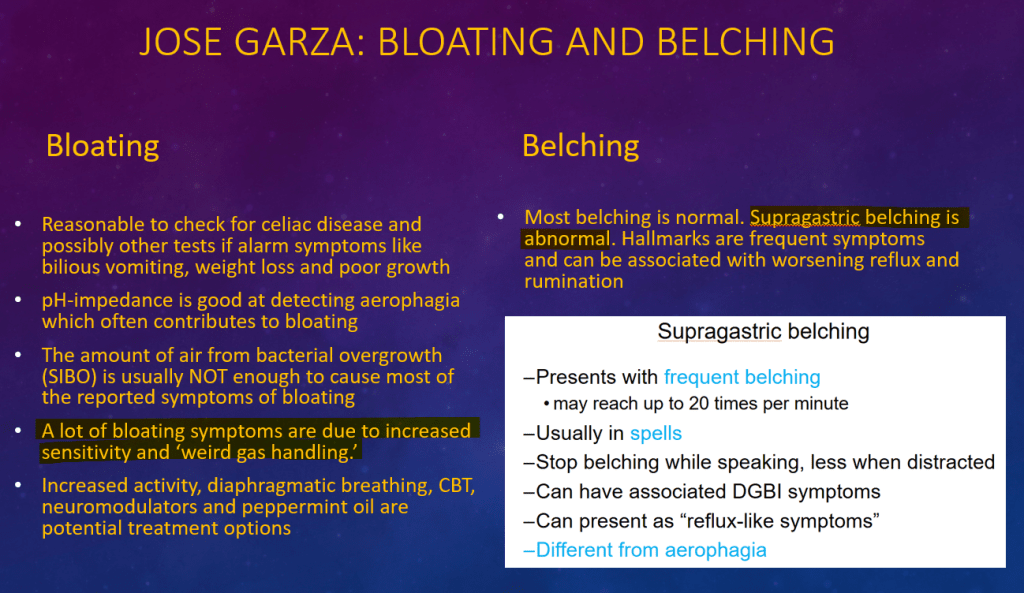
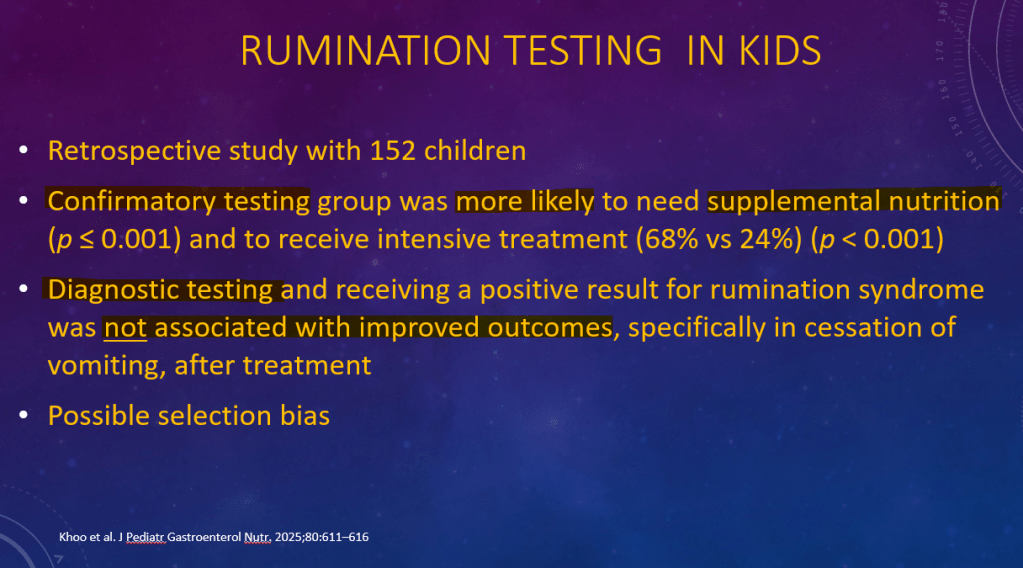
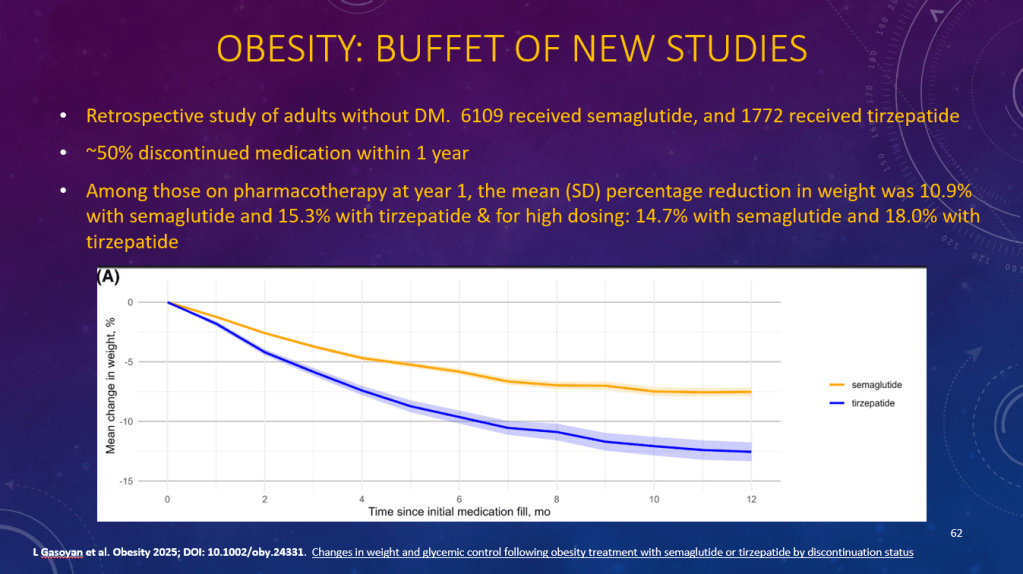
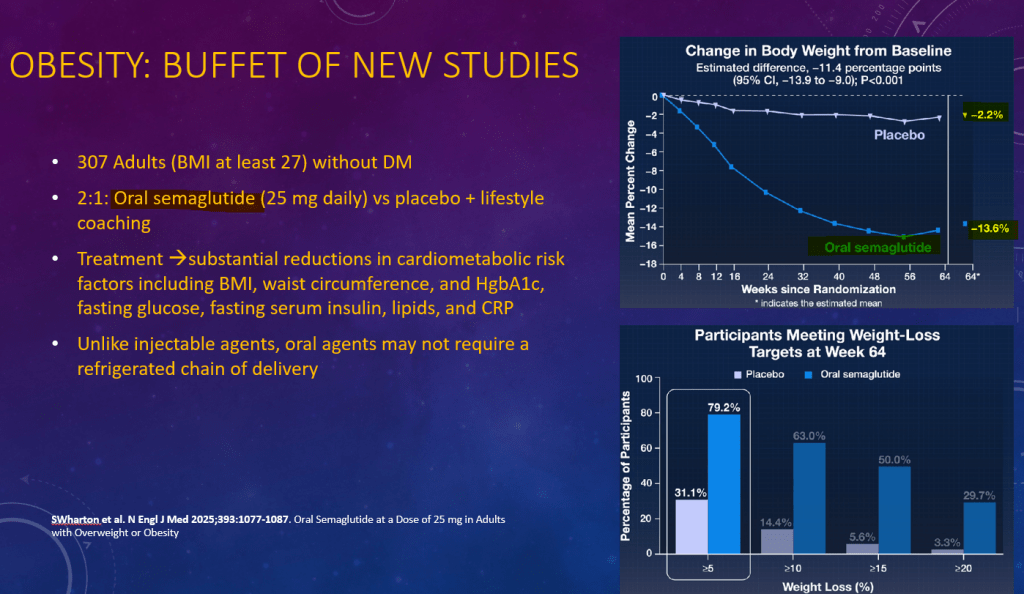
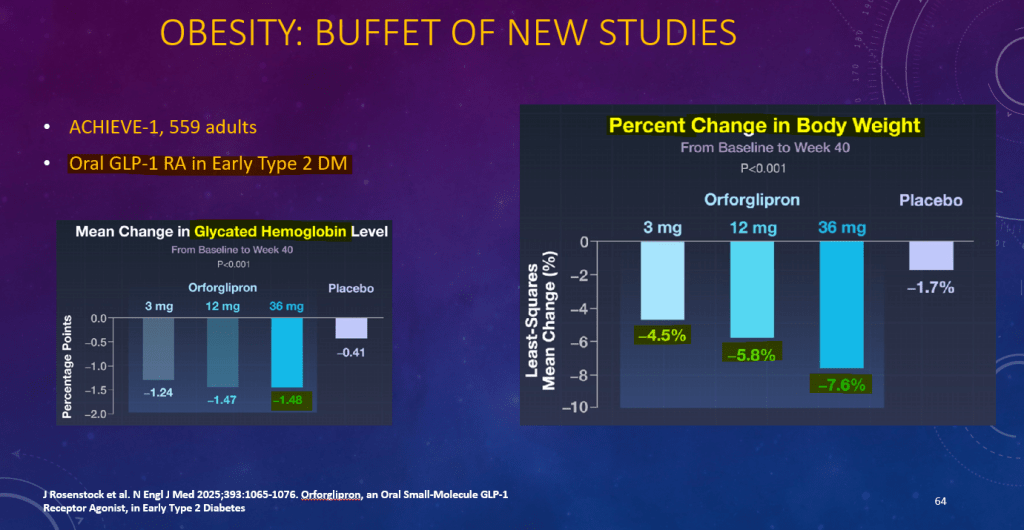
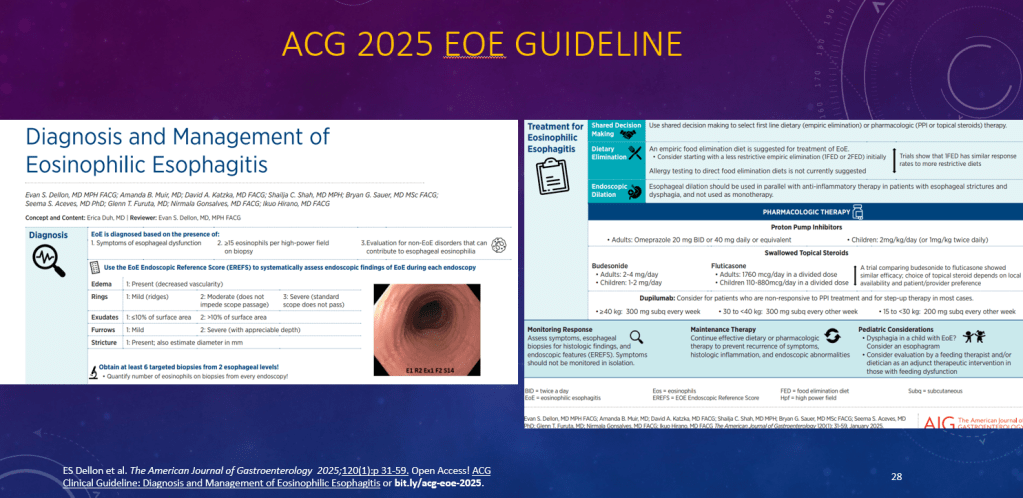
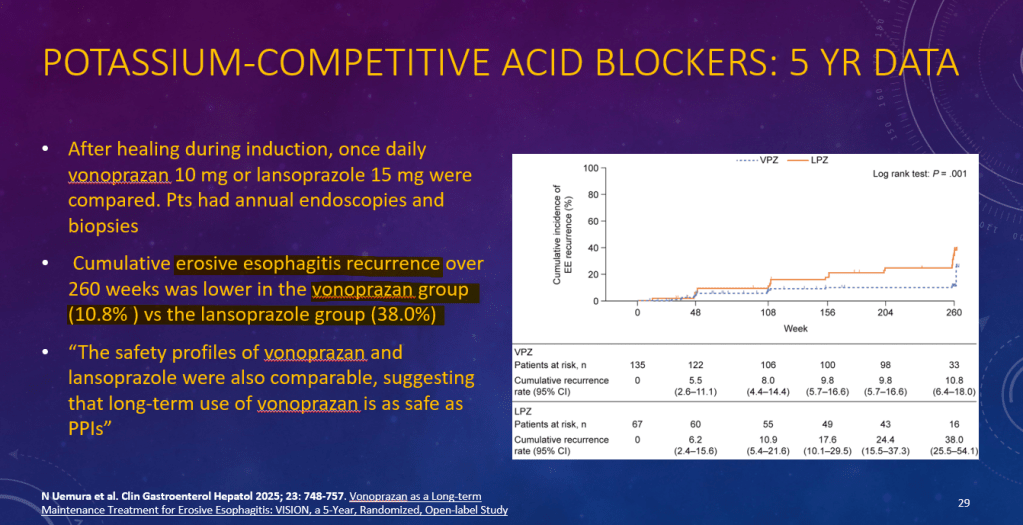
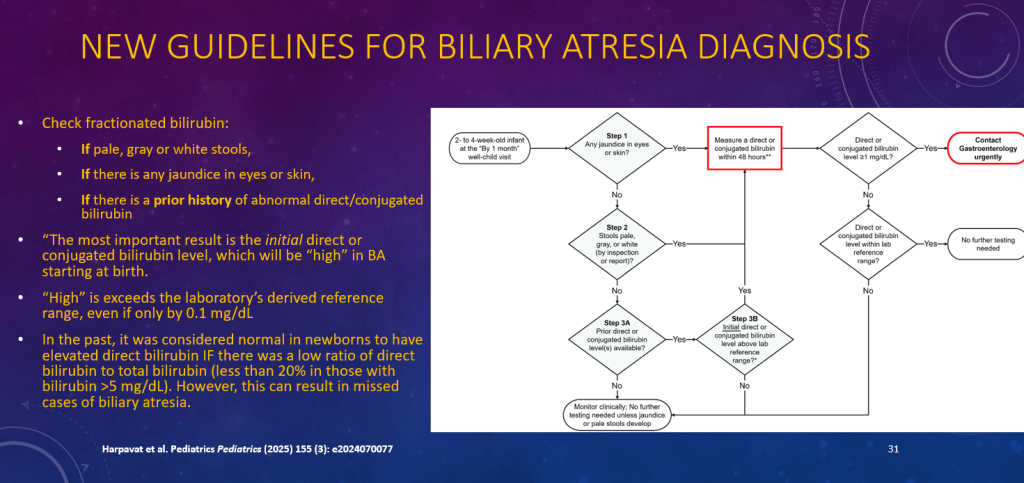
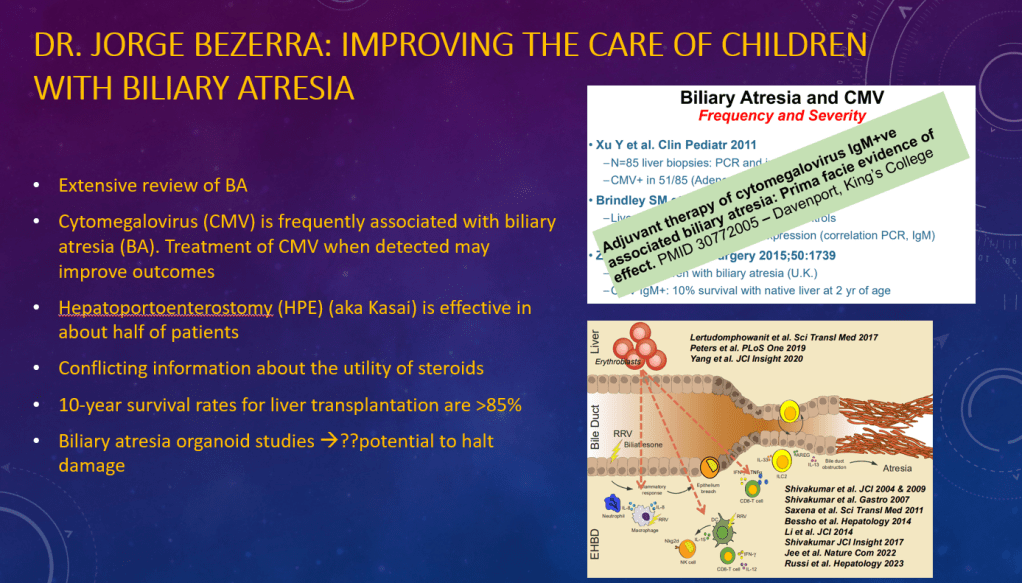
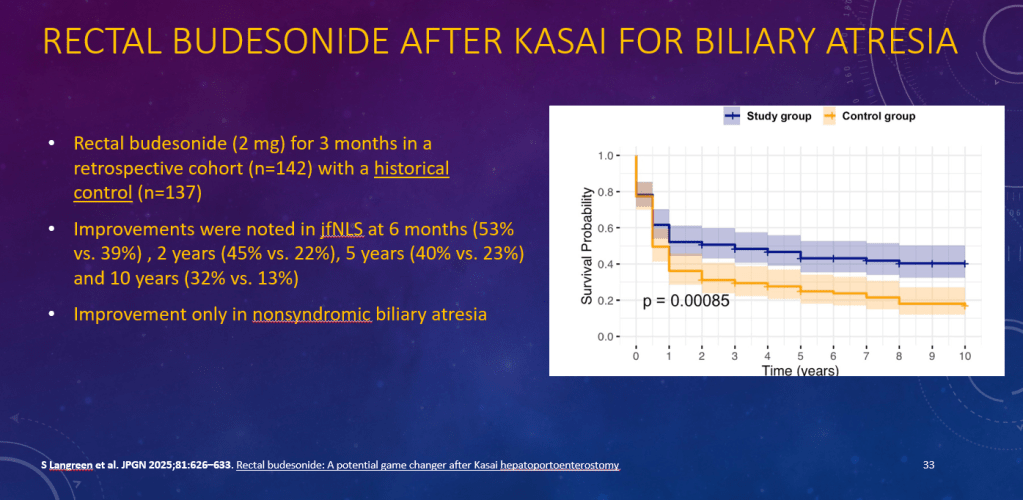
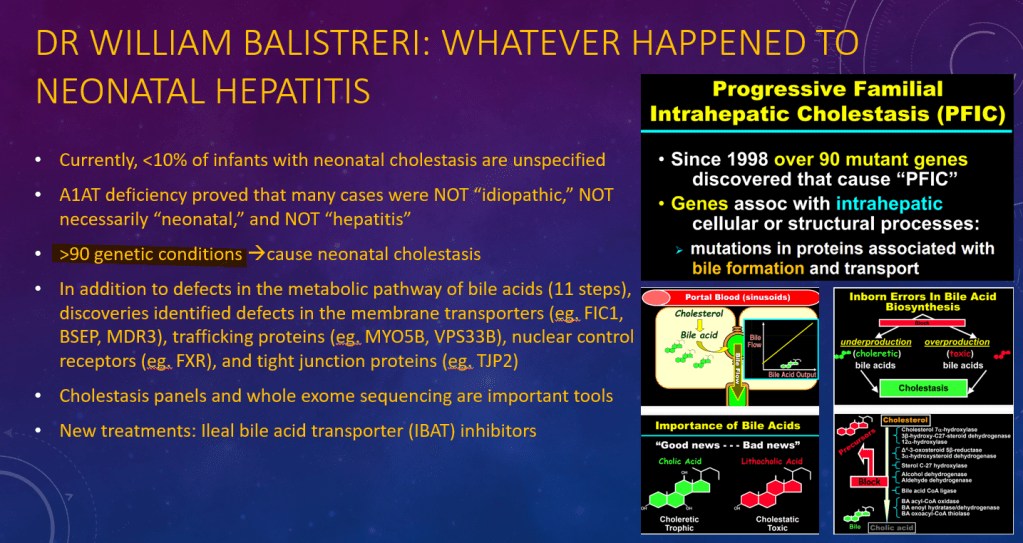
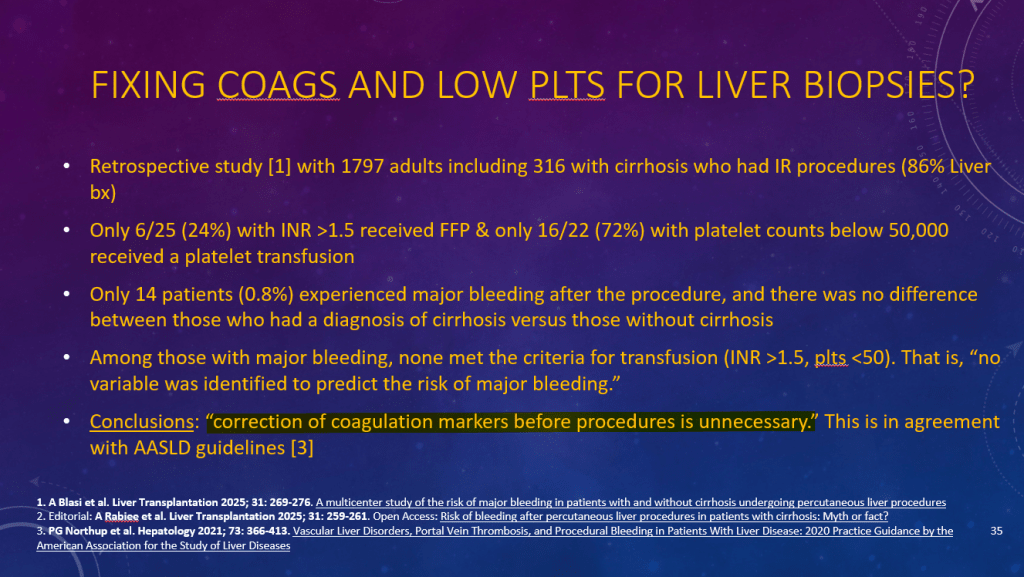
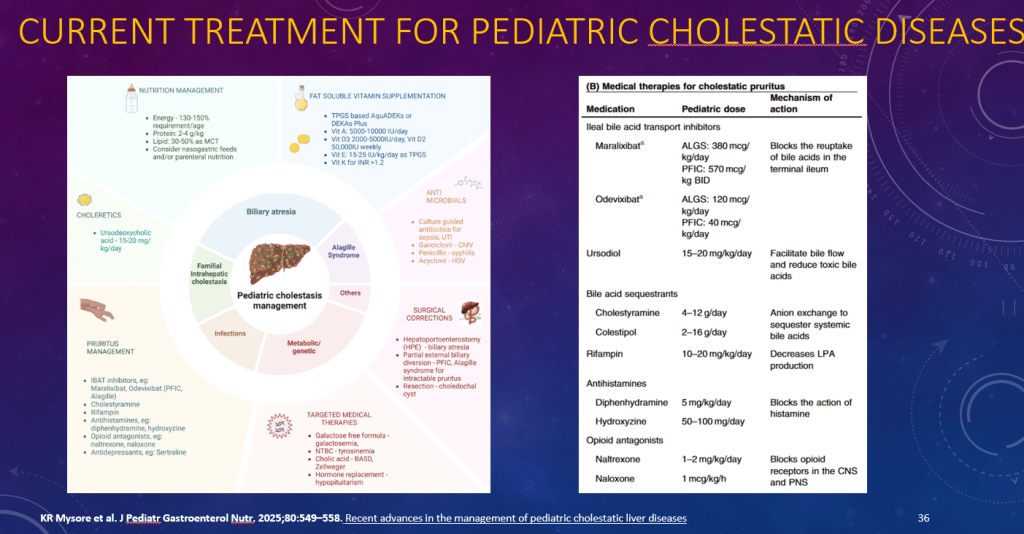
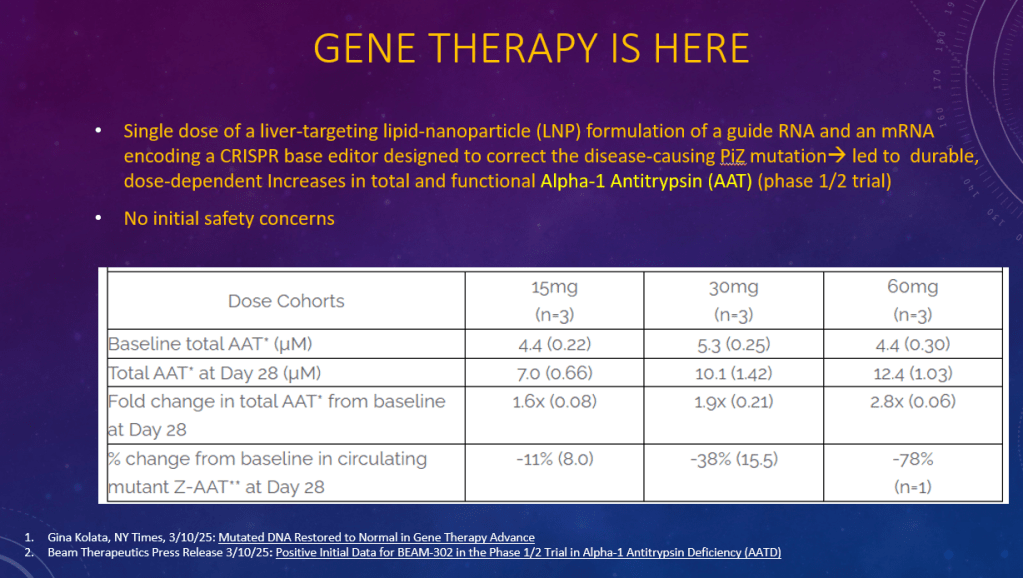
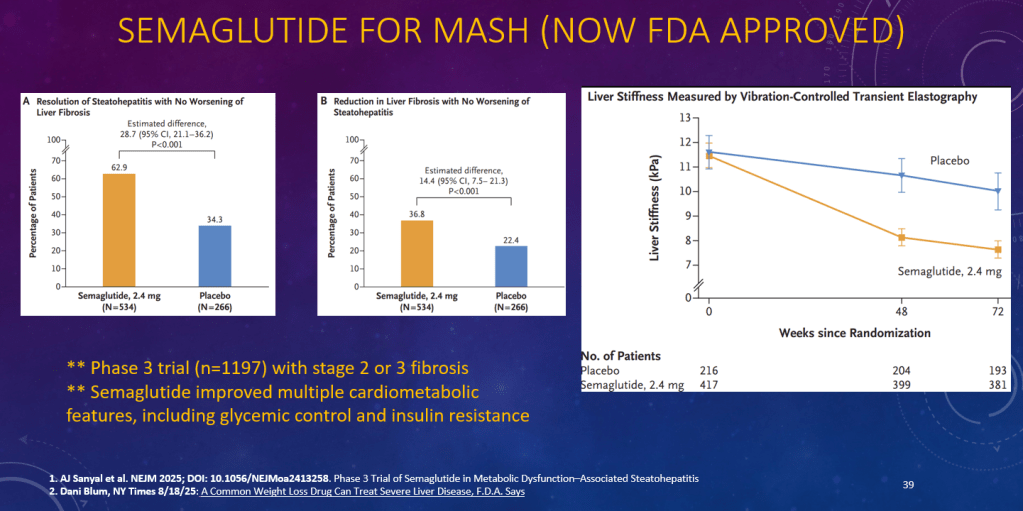
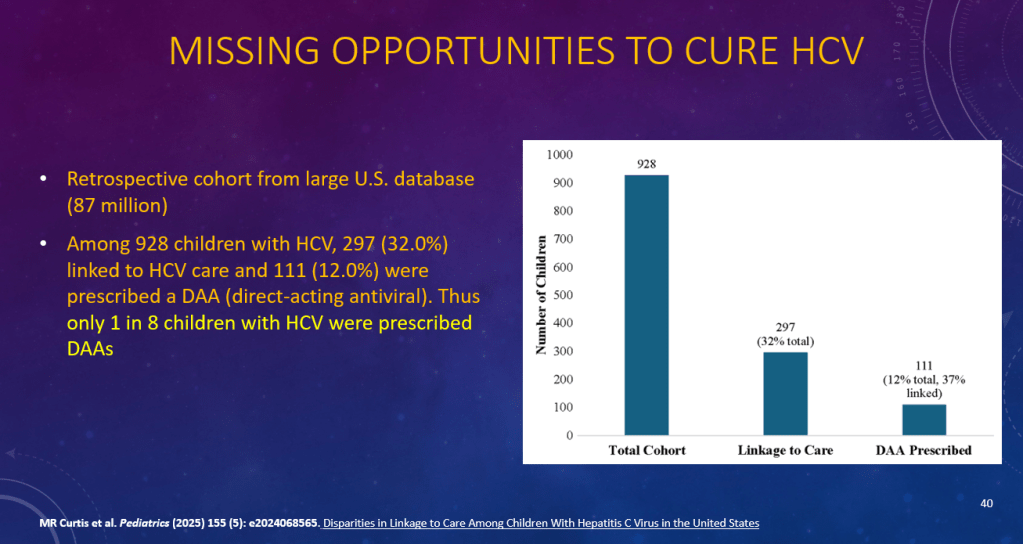
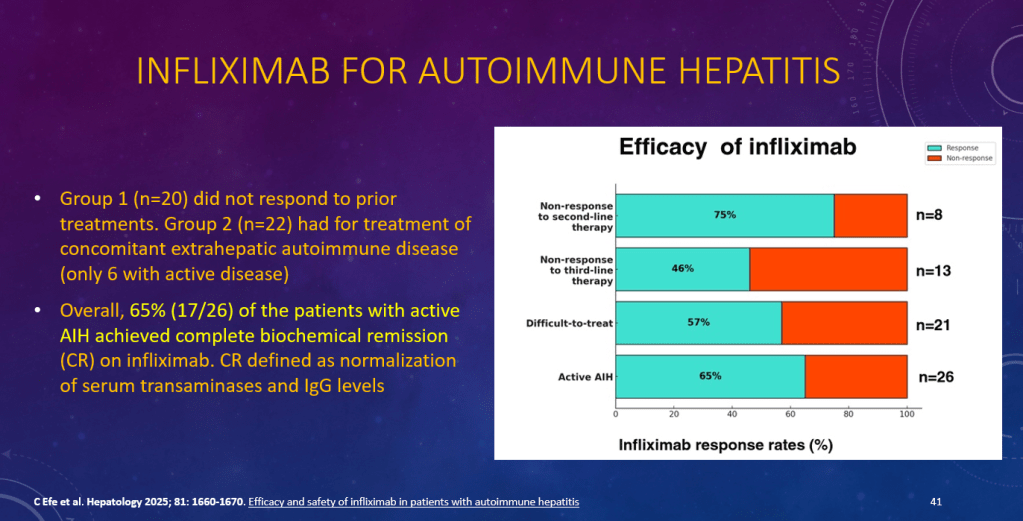
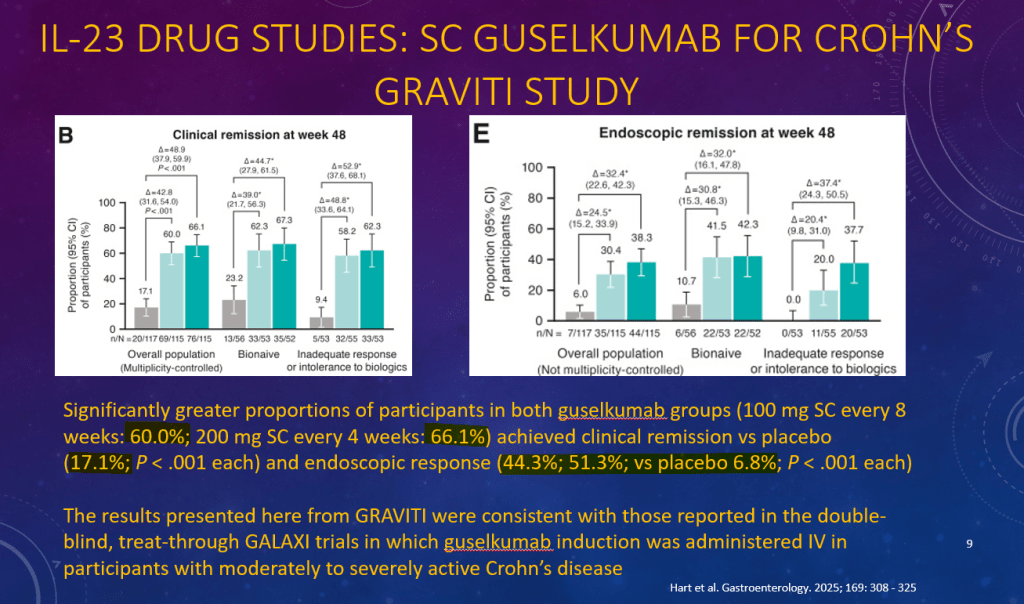
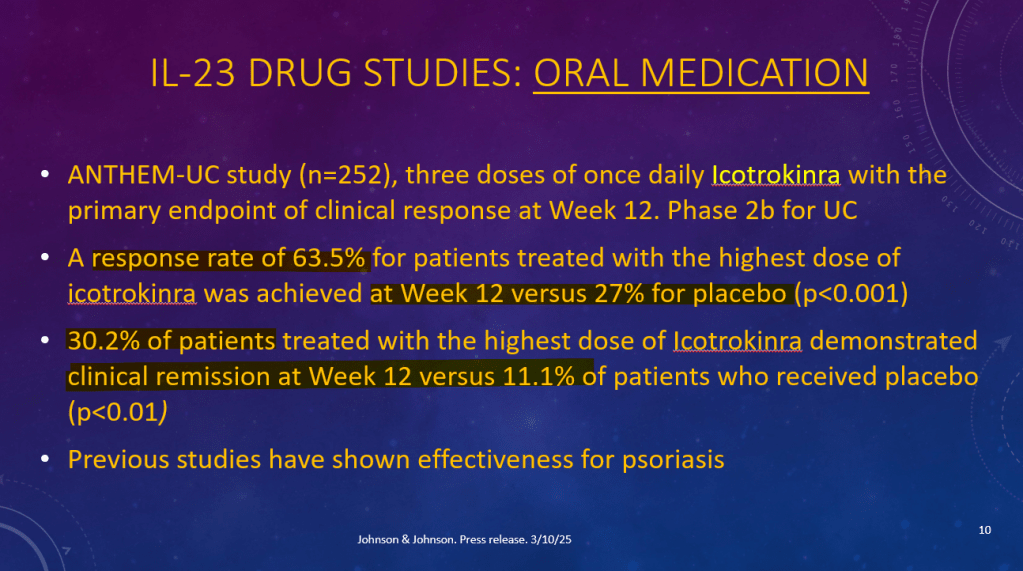
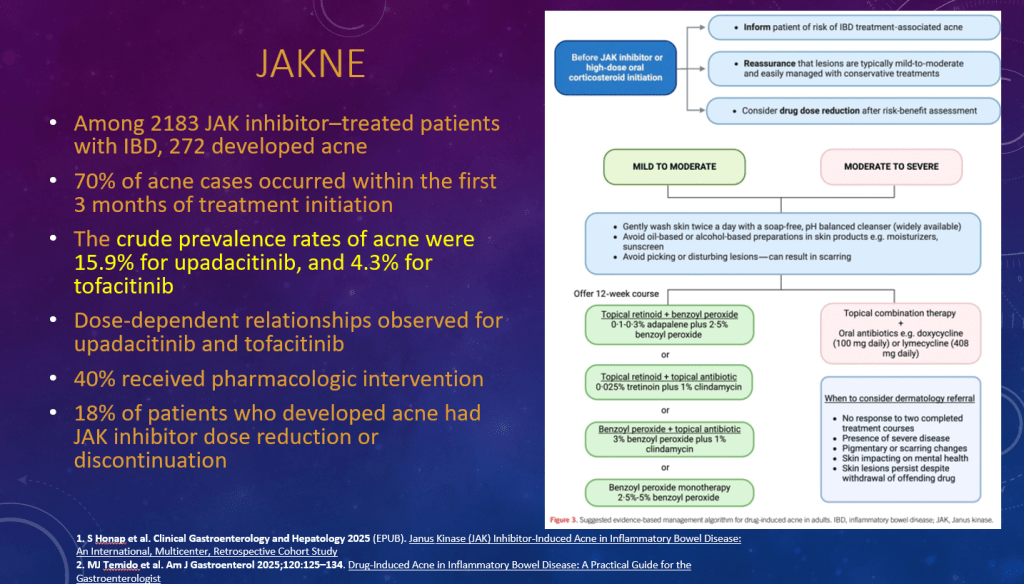
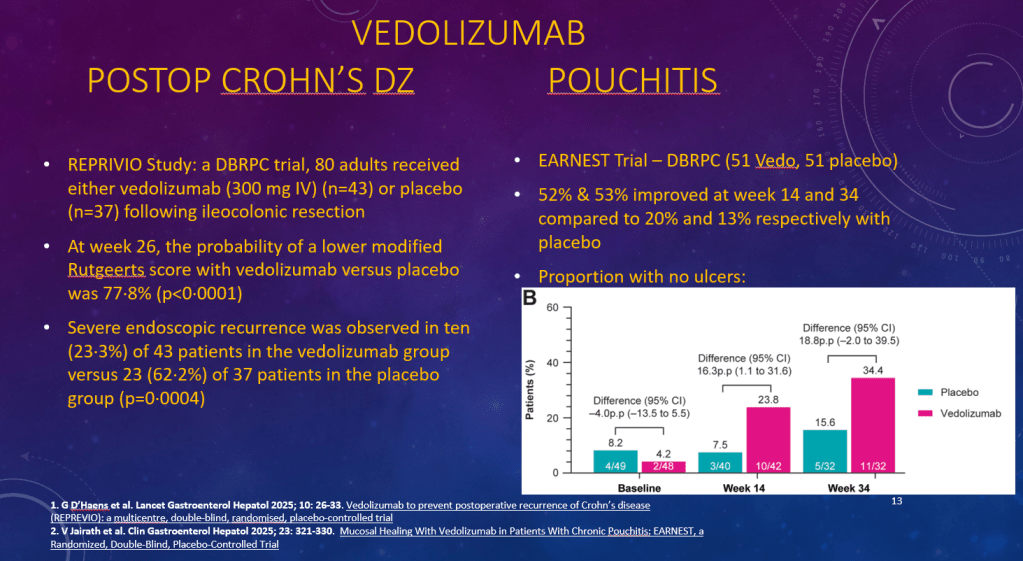
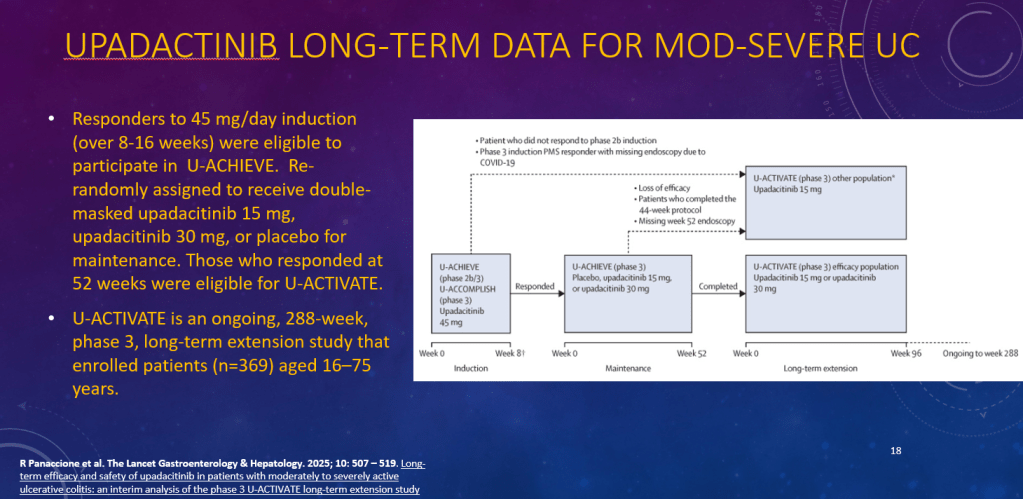
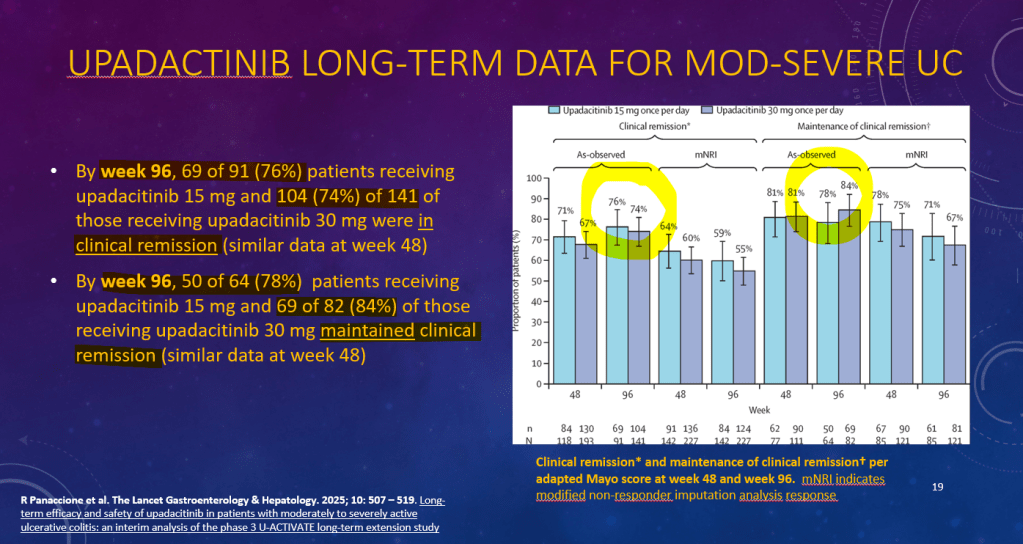
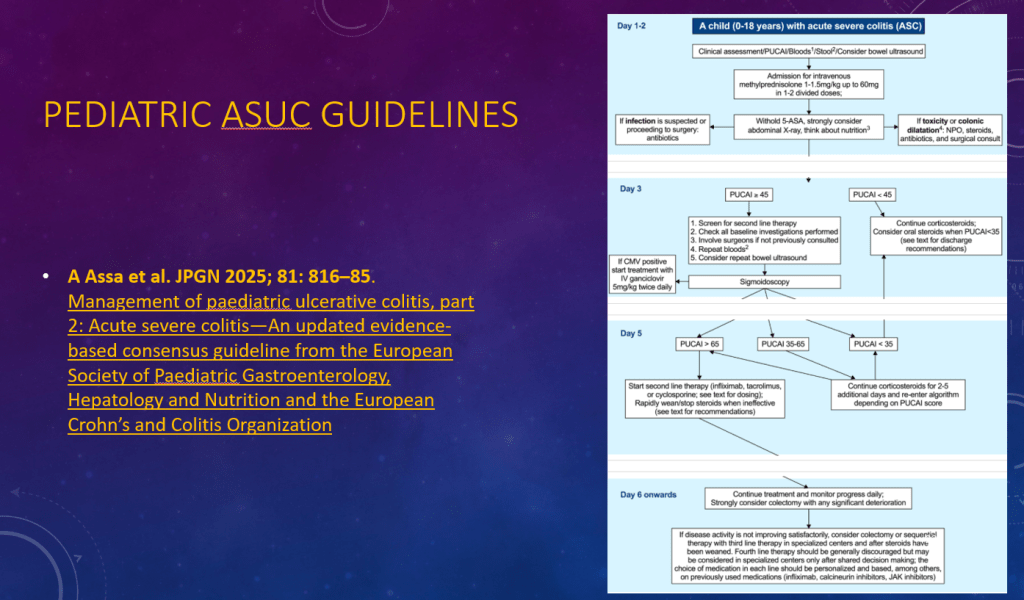
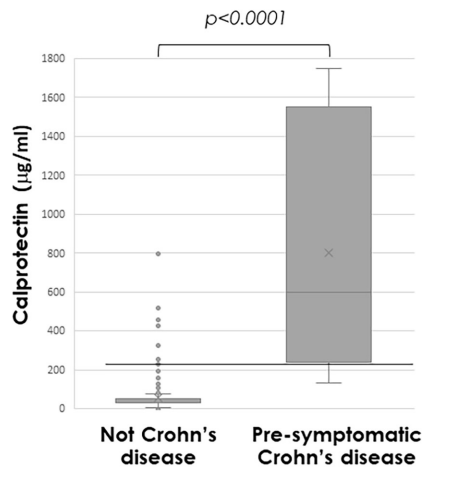
K Raymenants et al. Clin Gastroenterol Hepatol 2026; 24: 81-91. Diagnosis of Retrograde Cricopharyngeus Dysfunction Using High Resolution Impedance Manometry and Comparison With Control Subjects
Methods: Retrospective analysis of High Resolution Impedance Manometry
(HRiM) with belch provocation was performed between May 2021 and April 2024 in 55 patients with R-CPD, 30 control patients, and 15 healthy volunteers. Age of patients with R-CPD was 22-35 years.
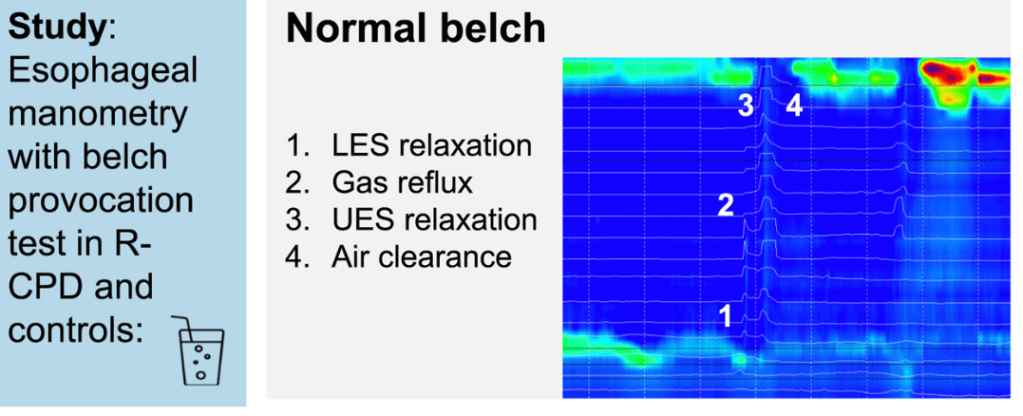
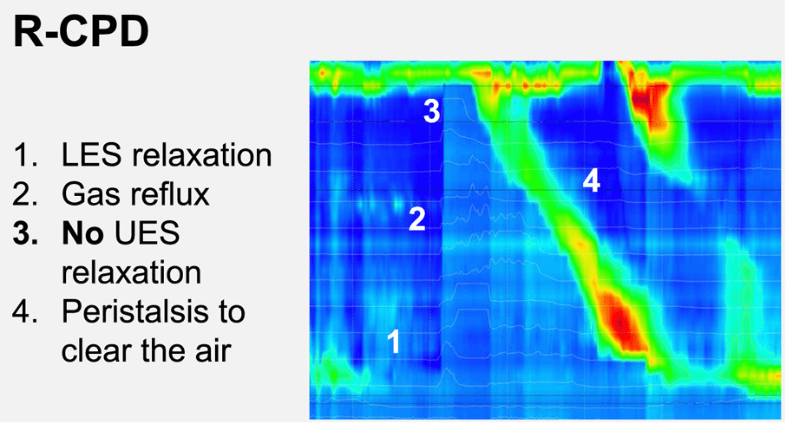
Key findings:
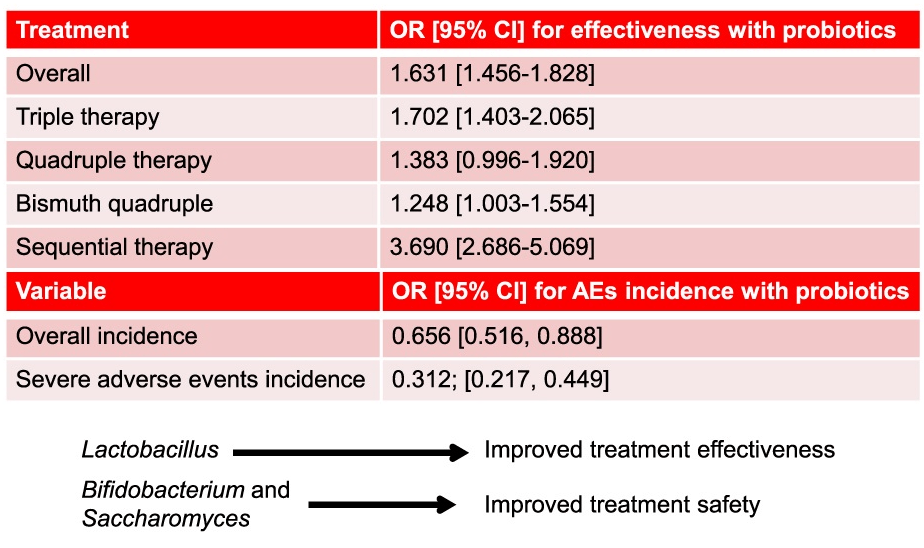
- During belching, we saw higher UES pressures in R-CPD patients vs controls, leading to incomplete air clearance and air oscillating in the esophagus (P < .0001)
- After BT injection, median UES pressures during belching decreased (56 vs 3 mmHg), and air clearance improved (P < .0001)
- A maximum UES pressure during belching >31 mmHg adequately discriminated patients from controls
- Interestingly, the authors did not include one of the major findings in their abstract: “Symptom improvement of at least 50% was present in 57% of patients, which is lower than reported up to now”
- R-CPD patients had inability to belch in 100%, gurgling chest noises in 100%, bloating in 92%, chest pain in 67%, nausea in 59%, and heartburn (at least weekly) in 65%
My take: Recognition of this treatable disorder is important. However, the lower improvement rate in this study is useful for counseling patients. My suspicion is that this finding likely reflects more widespread results as initial studies could have more selection or reporting bias.
Related blog posts: