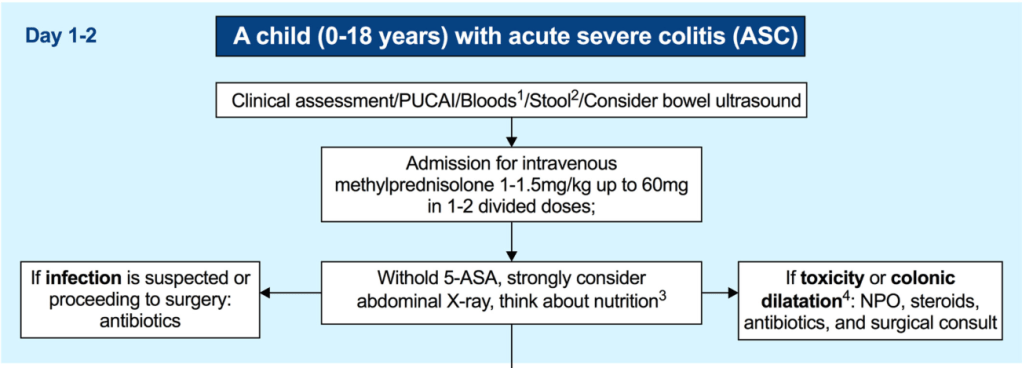
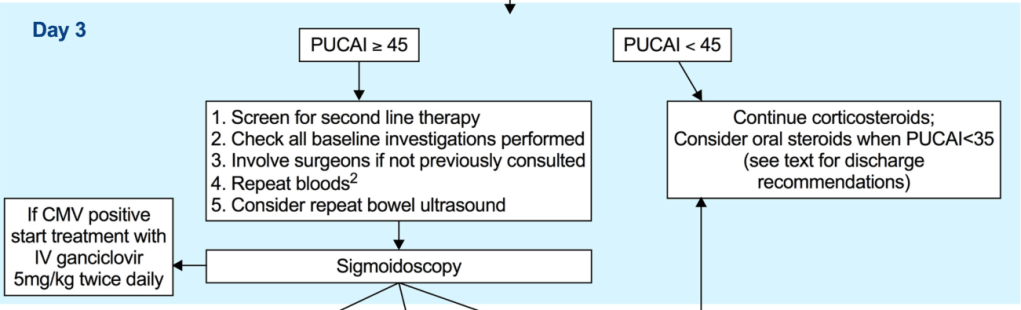
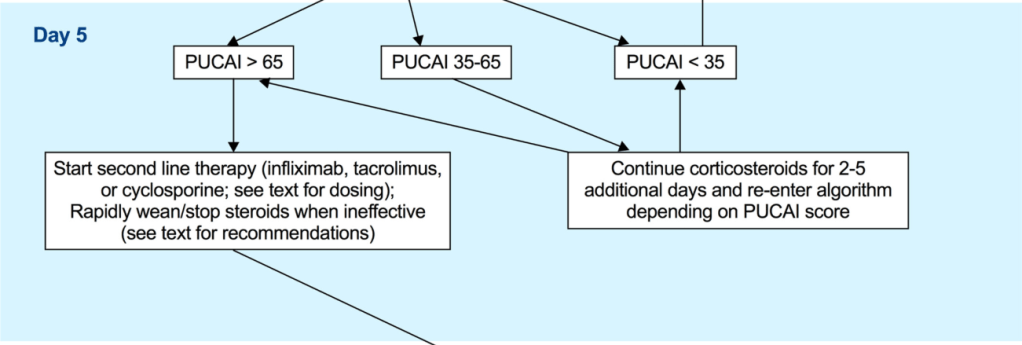
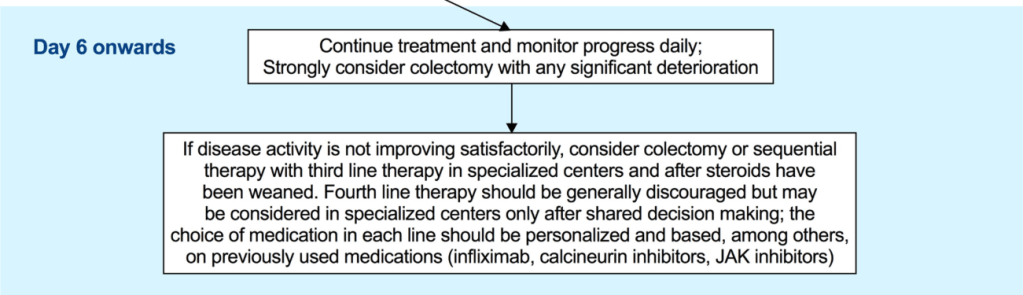
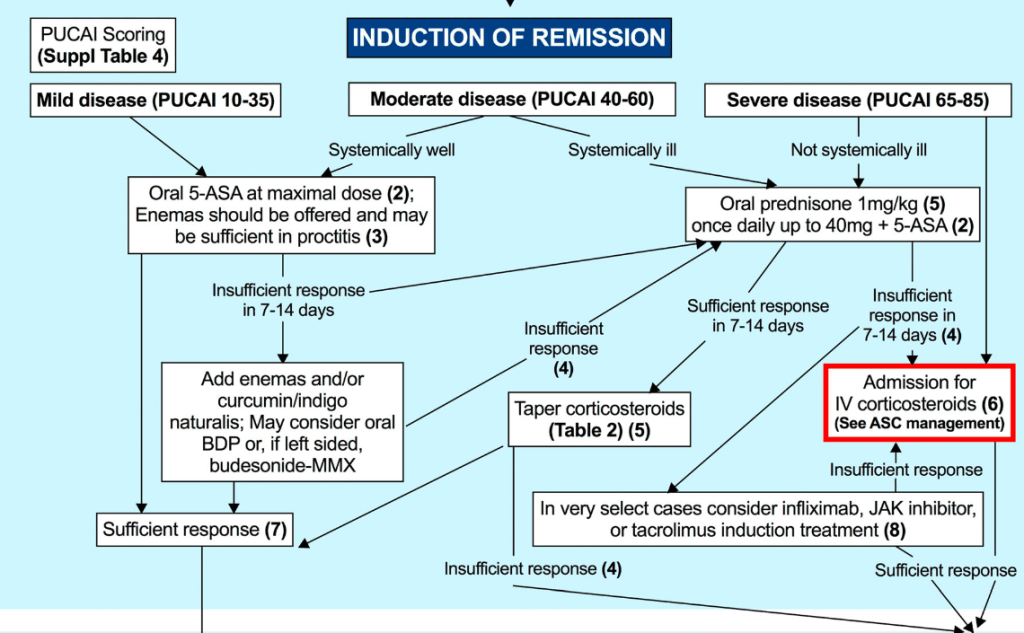
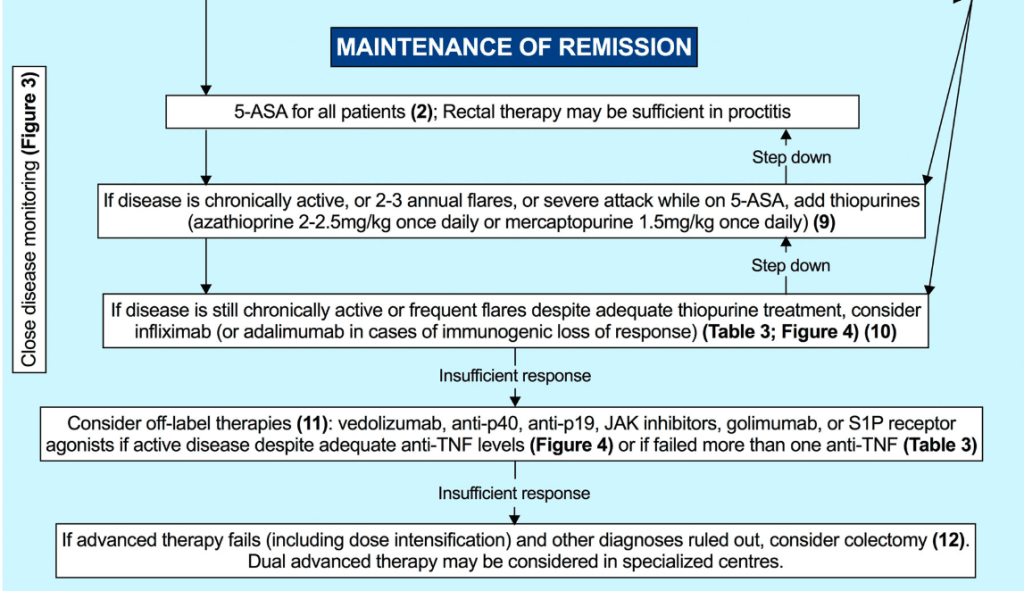
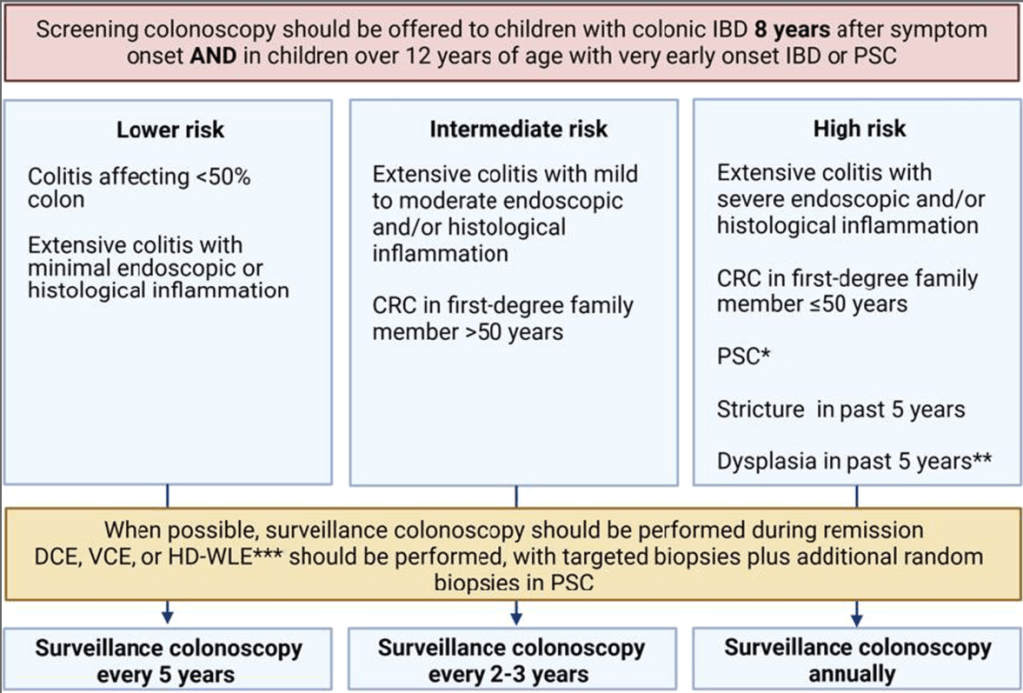
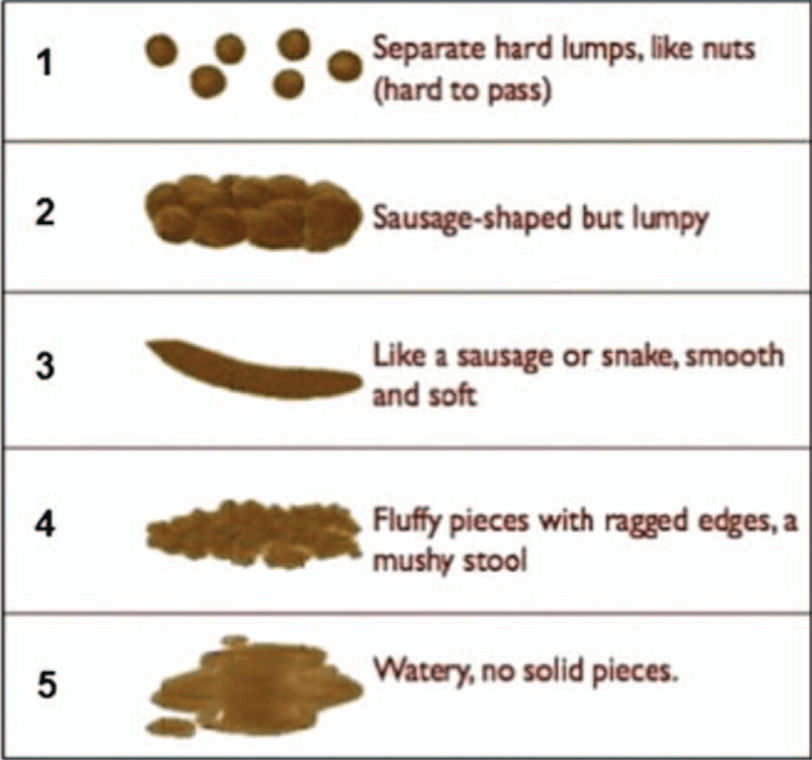
EJ Figueroa et al. Am J Gastroenterol 2025;120:1941–1945. A Practical Guide to Diet and IBD
Background: “A growing body of evidence suggests that dietary intake may play a role in the pathogenesis and perpetuation of IBD-associated inflammation. Human and animal-based studies have identified various dietary components, such as meat and artificial
food additives, associated with intestinal inflammation.”
“Despite the interest of patients in dietary therapy, robust data surrounding the potential harms and benefits are limited (1). Patients often attribute symptoms of IBD to their dietary intake and will avoid foods they perceive as triggers. Overly restrictive diets can lead to decreased food-related quality of life, malnutrition, or micronutrient deficiencies.”
Key points:
- “For patients seeking general guidance, we recommend avoiding ultra-processed foods, artificial thickeners, and sweeteners and trying to adhere to a predominantly plant-based diet focusing on fruits and vegetables, with moderate amounts of lean proteins, and for patients with UC, reduced amounts of red meat.”
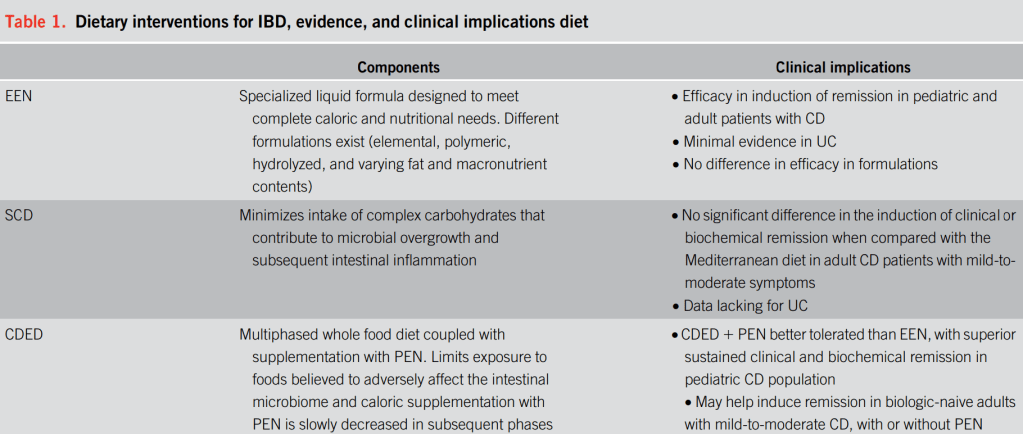
- For Crohn’s disease: “the highest quality evidence supporting dietary management of IBD is for EEN and PEN in mild-to-moderate CD.”
- “For patients seeking specific guidance, …there is good evidence for the use of EEN and
PEN with CDED for induction of remission in Crohn’s disease…short-duration use of EEN/PEN can be offered to patients with medically refractory disease who require a bridge to their next advanced therapy.” - For Crohn’s disease: “Dietary strategies using whole food alone can improve gastrointestinal symptoms but have not definitively demonstrated successful control of inflammation. Their use is generally recommended for patients with mild-to-moderate symptoms.”
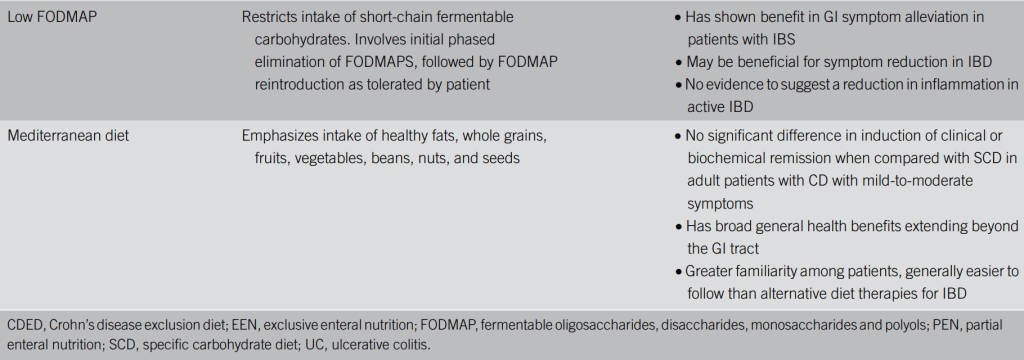
- For Ulcerative Colitis: “There remains insufficient evidence to support the use of dietary approaches in the management of UC, but some evidence suggests a diet high in fiber and low in fat may be of benefit.”
Table 1 provides a good summary:
Other points:
Formula for EEN: “A Cochrane meta-analysis found no difference in efficacy between polymeric or elemental EEN formulations…Because of lower cost and better palatability, polymeric formulas are most often used in clinical practice.”
SCD Diet vs Mediterranean:”Iin a randomized superiority trial that compared 6 weeks of the SCD with a Mediterranean diet in adults with Crohn’s disease with mild-to-moderate symptoms, the SCD was not found to be superior to the Mediterranean diet (14). After 6 weeks of therapy, less than half the participants (46.5% on SCD and 43.5% on the
Mediterranean diet) were in clinical remission, and neither diet resulted in normalization of C-reactive protein concentration for most patients.”
My take: This article provides a good summary of the current evidence supporting the role of dietary treatment for IBD. In patients interested in specific diets, the assistance of a nutritionist/dietician is very important.
Related blog posts:
- AGA Guidance: Nutritional Therapies for Inflammatory Bowel Disease
- The Quality of Evidence for Dietary Treatments in Inflammatory Bowel Disease
- CDED + PEN: An Alternative Diet to Exclusive Enteral Nutrition?
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- More Evidence That A Proinflammatory Diet May Increase the Risk of Crohn’s Disease
- Pushing the Boundaries on Dietary Therapy for Crohn’s Disease (CD-TREAT)
- Predicting Enteral Nutrition Therapy Response in Patients with IBD
- Dietary Therapy for Adults with Crohn’s Disease
- Trial by Diet Approach for Crohn’s Disease in Children
- Dietary Therapy for Inflammatory Bowel Disease –Useful Update
- Mediterranean Diet vs Specific Carbohydrate Diet for Crohn’s Disease
- Mediterranean Diet’s Impact on Crohn’s Disease Outcomes