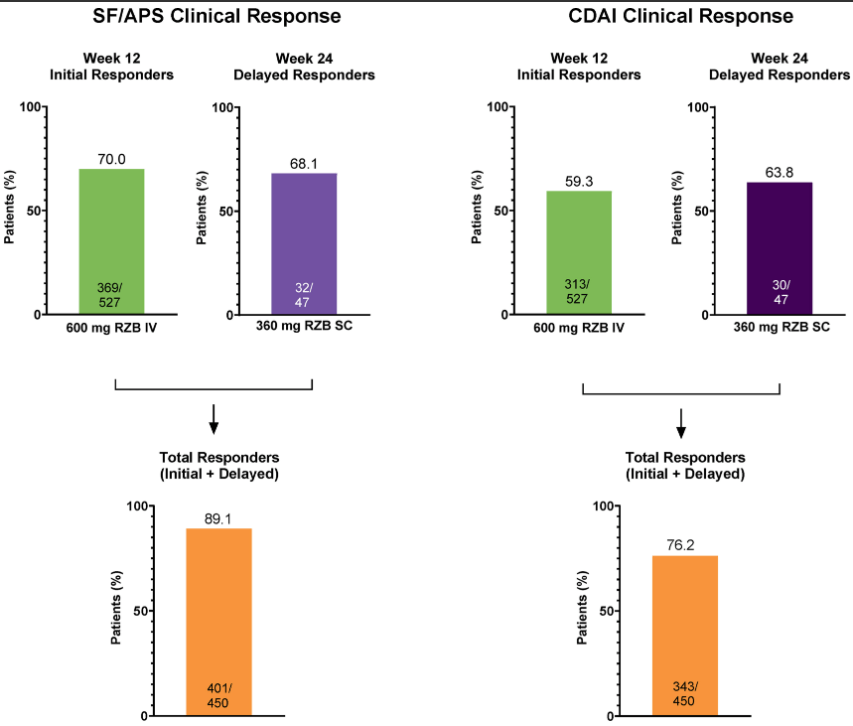
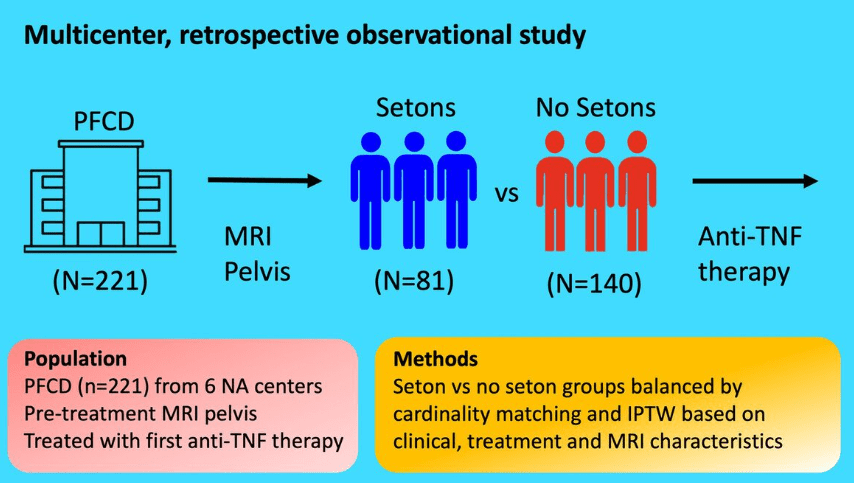
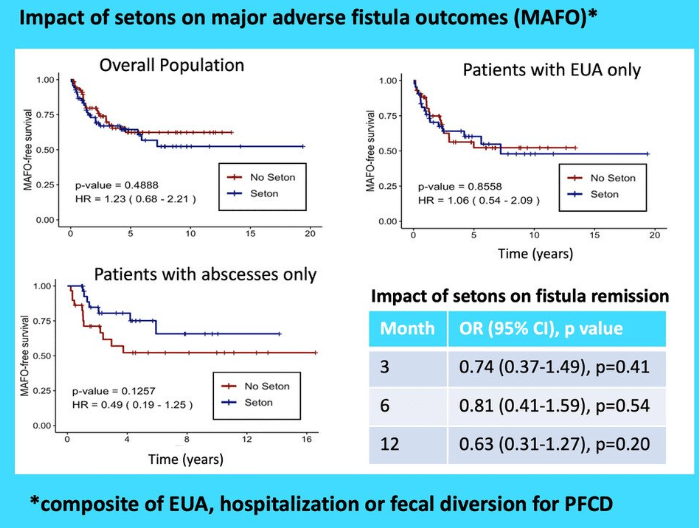
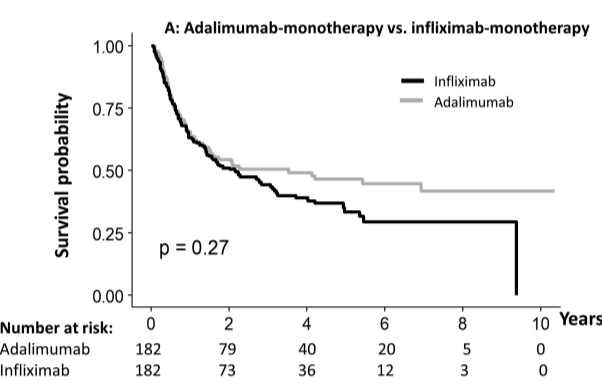
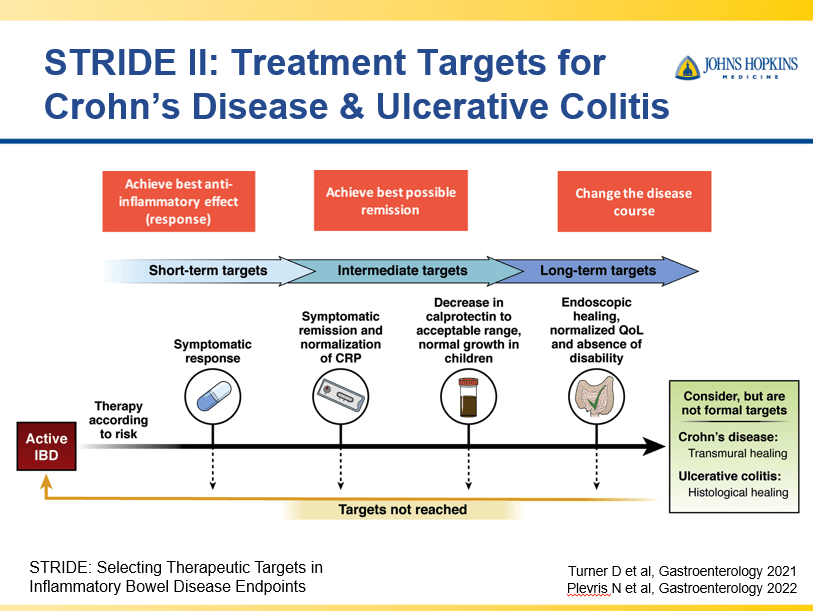
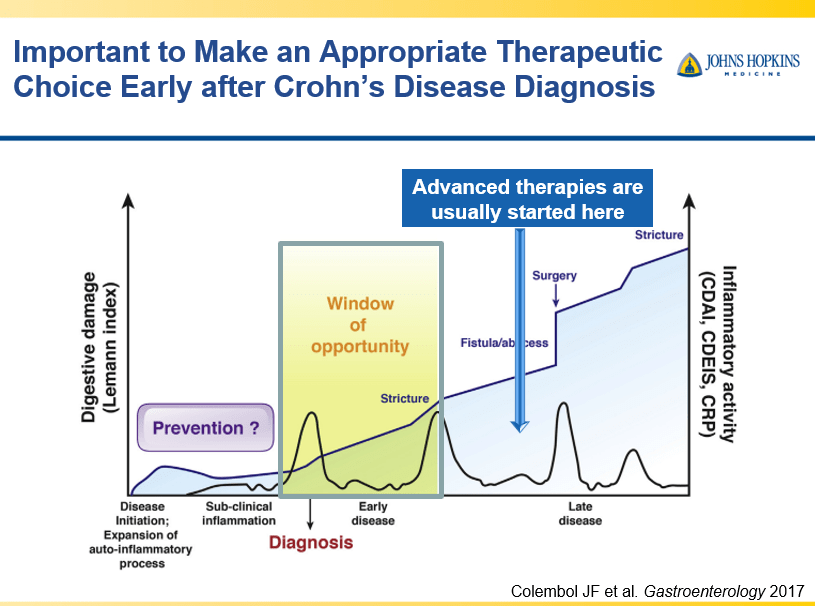
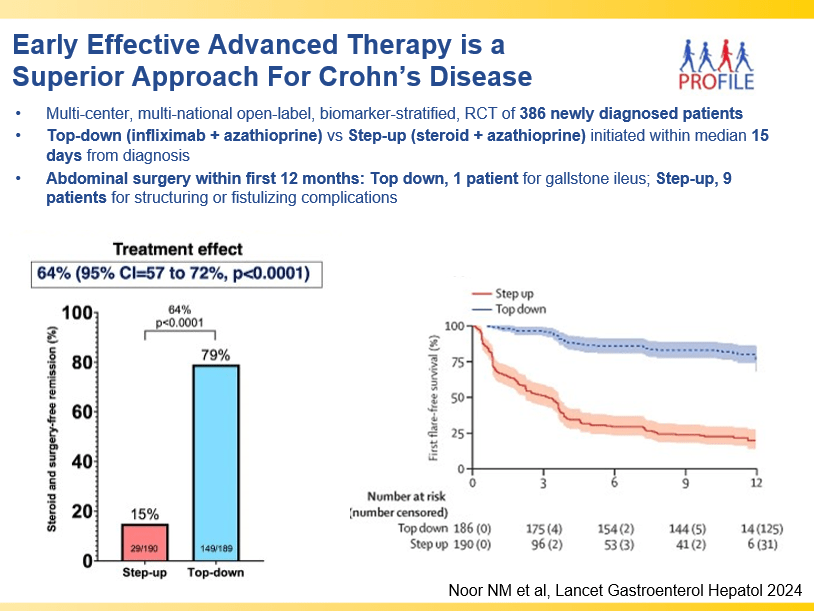
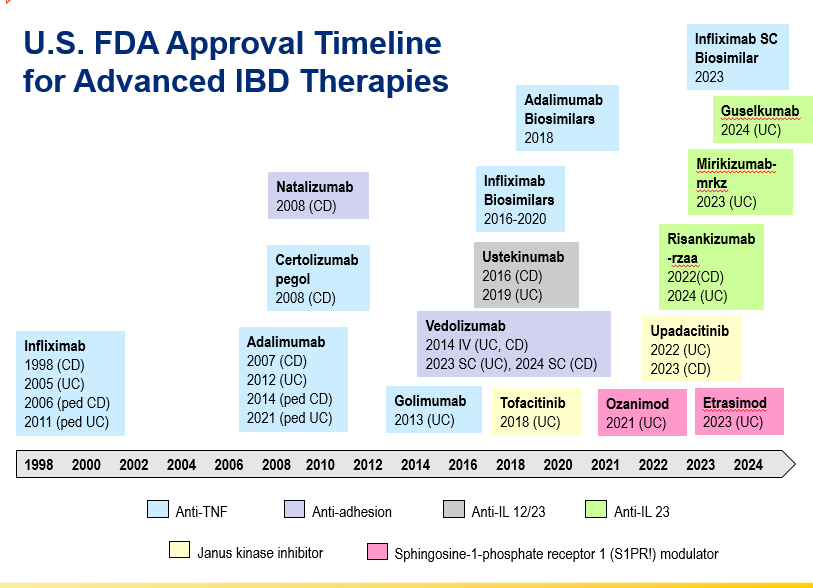
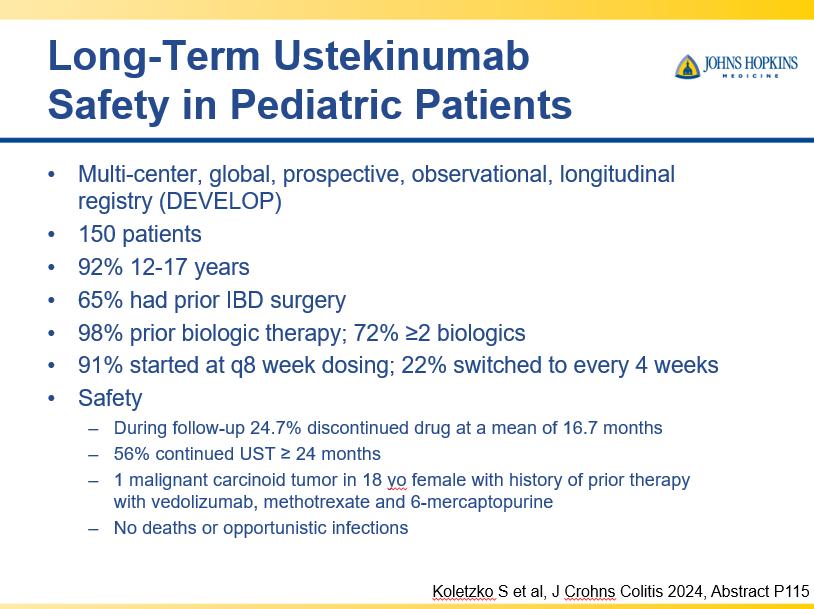
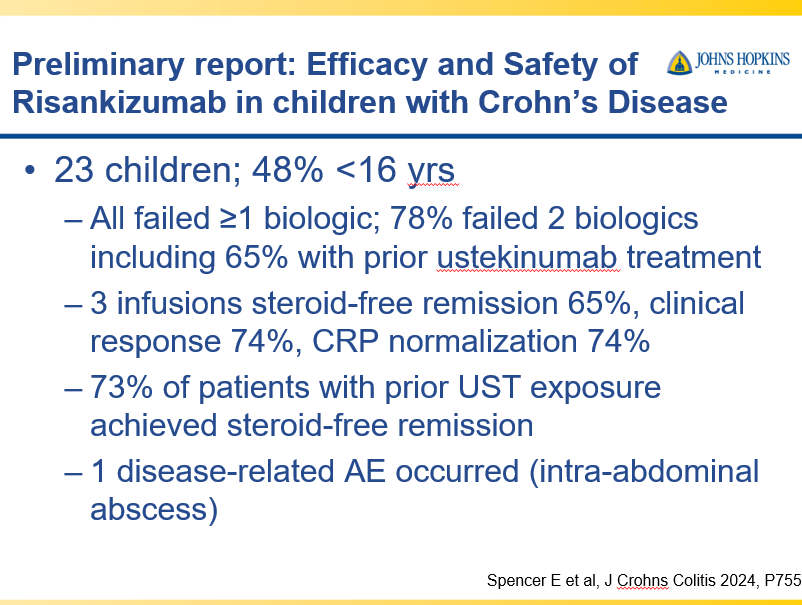
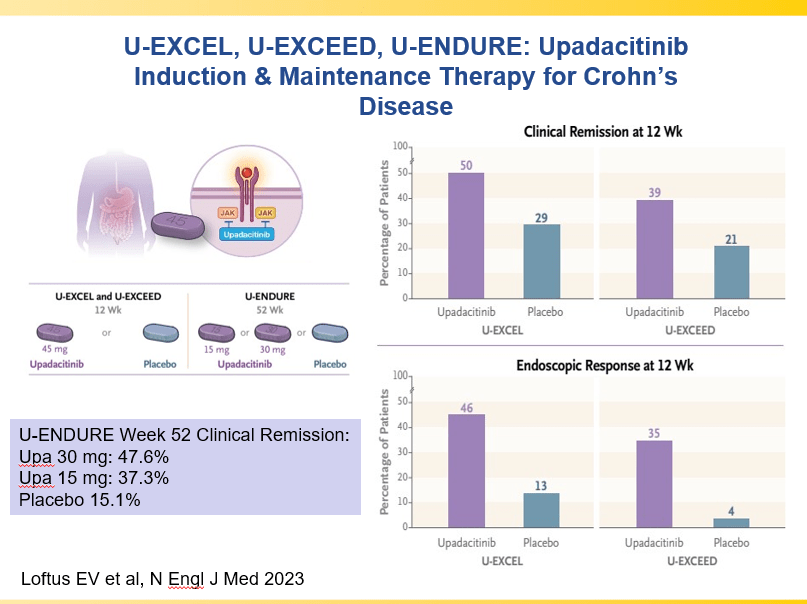
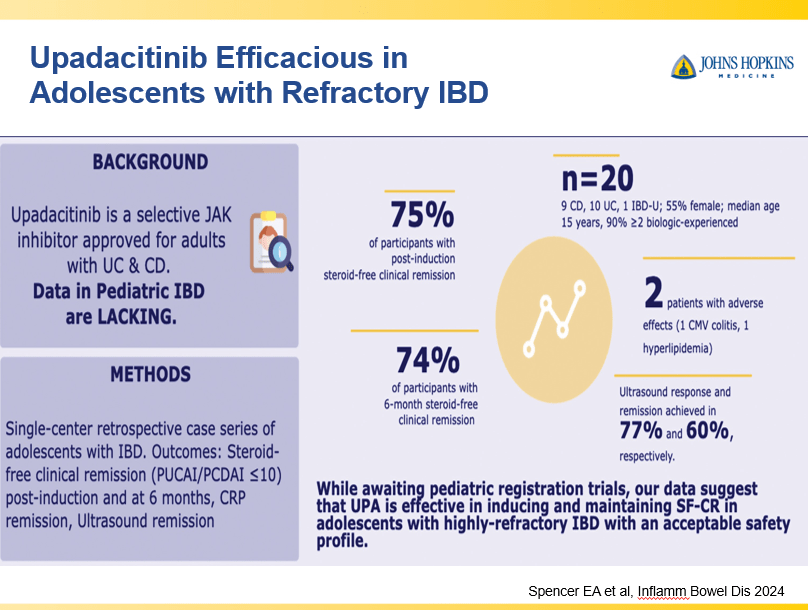
RS Dalal et al. Clin Gastroenterol Hepatol 2025; 23: 662-664. Outcomes After Dose Intensification of Risankizumab for Crohn’s Disease
This retrospective study reviewed adults with Crohn’s disease (CD) who underwent dose intensification of maintenance from 360 mg every 8 weeks to every 6 weeks (n=11) or every 4 weeks (n=11).
Key findings:
- Median time to first intensified dose was 228 days
- Harvey Bradshaw Index (HBI) improved from a mean of 7.1 to 4.3 after 8 to 16 weeks
- There was also improvement (not statistically significant) in mean CRP (1.64–>0.42 mg/dL) and mean calprotectin (774 –>650 mcg/g)
- At 8 to 16 weeks, 64% (14 of 22) had a clinical response, 45% (10 of 22) achieved steroid free clinical remission
My take: This small study suggests that the majority of patients with a loss of response to standard dosing can be recaptured with dose intensification.
Related blog posts:
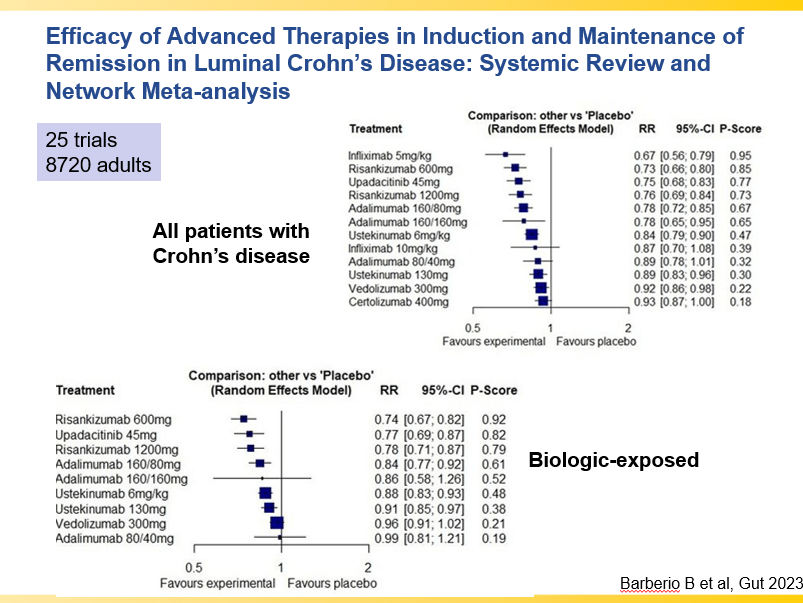
- Impressive Results for Risankizumab in Refractory Crohn’s Disease
- Risankizumab for Ulcerative Colitis
- Crohn’s Disease: Risankizumab Real-World Data
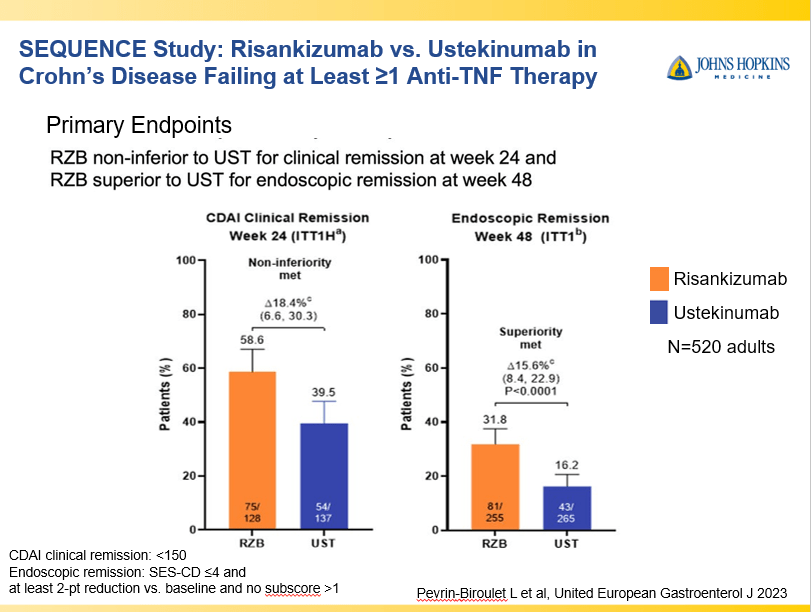
- Risankizumab Outperforms Ustekinumab
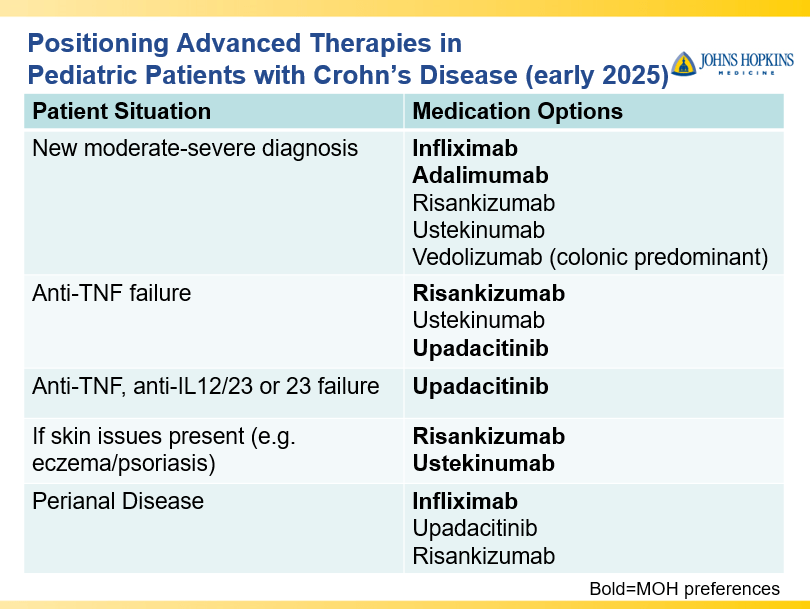
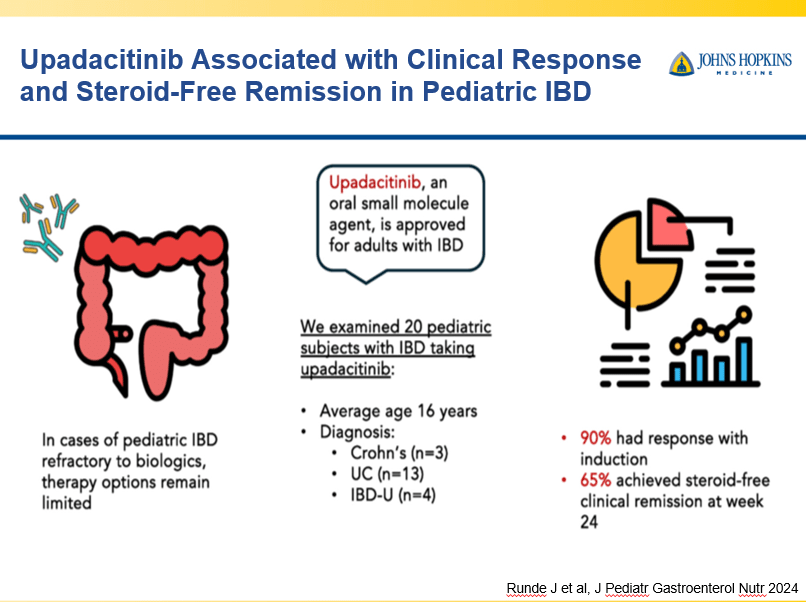
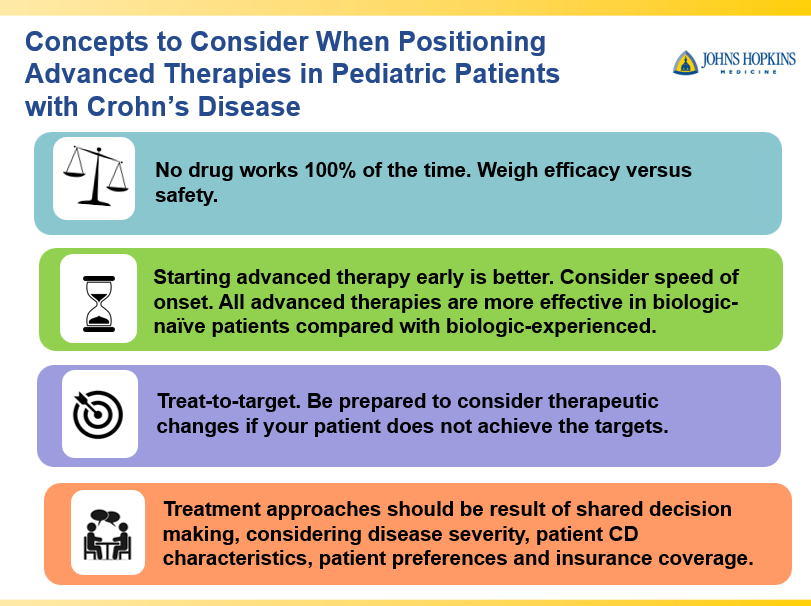
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease

Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.