7/1/24 FDA approves biosimilar Pyzchiva® (ustekinumab-ttwe), to be commercialized by Sandoz in US


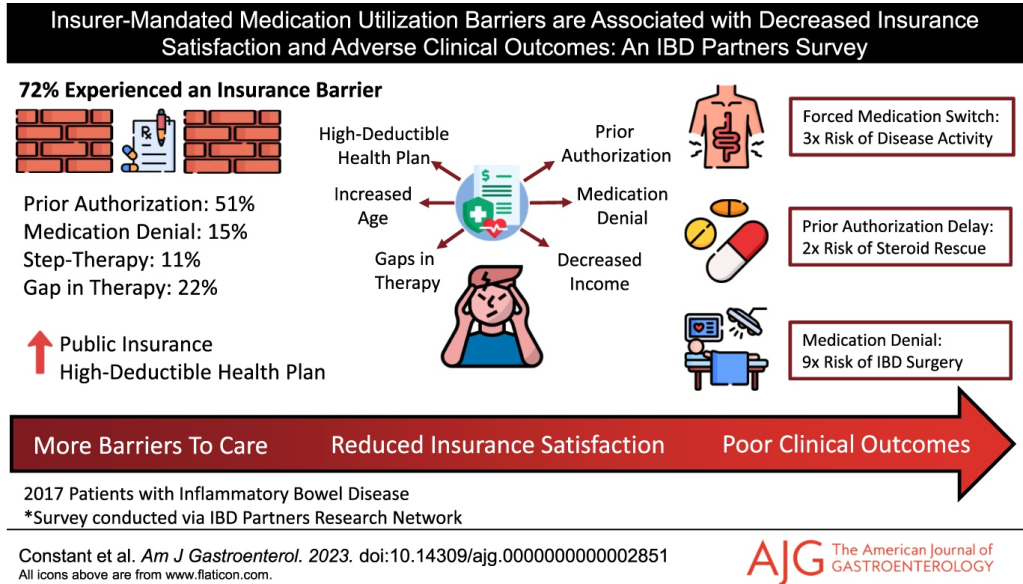
B Constant et al. AJG 2024; DOI: 10.14309/ajg.0000000000002851. Insurer-Mandated Medication Utilization Barriers are Associated With Decreased Insurance Satisfaction and Adverse Clinical Outcomes: An Inflammatory Bowel Disease Partners Survey
Key findings: In this longitudinal survey with 2017 patients, 72% experienced an insurer-mandated barrier, most commonly prior authorizations (51%). Fifteen percent were denied an IBD medication by their insurer, 22% experienced an insurance-related gap in therapy, and 8% were forced by their insurer to switch from an effective medication. Several insurance barriers were linked to negative downstream clinical outcomes, including prior authorizations associated with corticosteroid rescue (odds ratio [OR] 2.24]), forced medication switches associated with continued disease activity (OR 3.28), and medication denials associated with IBD-related surgery (OR 8.92).
Related blog posts:
S Danese et al. Lancet Gastroenterol Hepatol 2024; 9: 133-146. Efficacy and safety of 48 weeks of guselkumab for patients with Crohn’s disease: maintenance results from the phase 2, randomised, double-blind GALAXI-1 trial
In this phase 2 randomised, multicentre, double-blind trial with 309 adults, the authors report on the safety and efficacy of subcutaneous guselkumab maintenance regimens to week 48 in the GALAXI-1 study. Key findings:
Related blog posts:
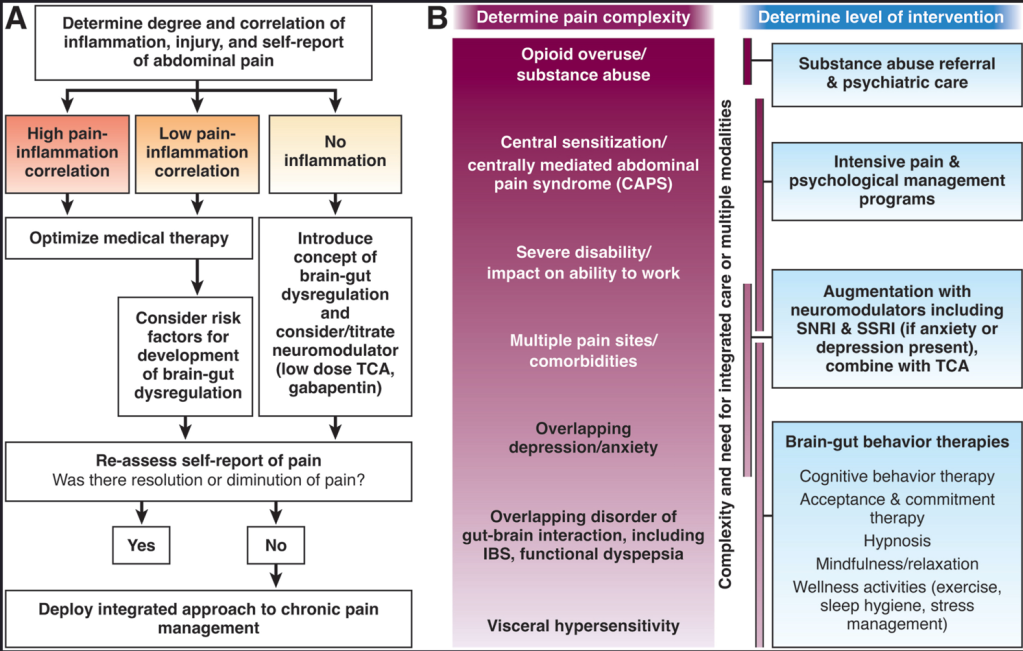
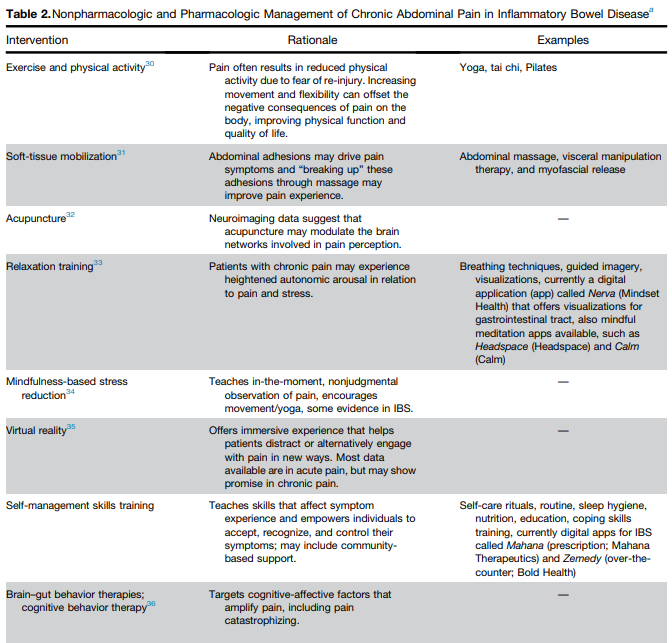
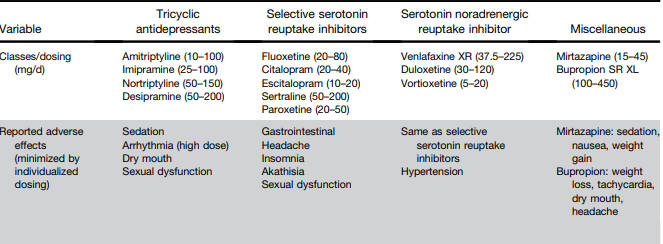
L Keefer et al. Gastroenterology 2024; 166: 1182-1189. AGA Clinical Practice Update on Pain Management in Inflammatory Bowel Disease: Commentary
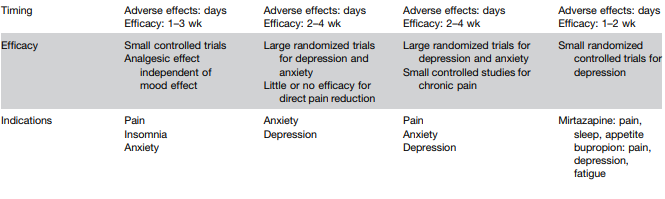
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
C Hernandez-Rocha et al. J Crohns Colitis 2024: 18: 615–627. Clinical Predictors of Early and Late Endoscopic Recurrence Following Ileocolonic Resection in Crohn’s Disease
Prospective Study n=365 adult patients with post-operative Crohn’s disease. Findings: At first colonoscopy, 109 [29.9%] had recurrence. Male gender (odds ratio [OR] = 1.95), non-White ethnicity [OR = 2.48], and postoperative smoking [OR = 2.78] were associated with recurrence, while prophylactic anti-TNF reduced the risk [OR = 0.28]. Postoperative anti-TNF prophylaxis had a protective effect on anti-TNF experienced patients but not on anti-TNF naïve patients. Among patients without recurrence at first colonoscopy, Rutgeerts score i1 was associated with subsequent recurrence [OR = 4.43]
A Lecoutour et al JPGN 2024; 78:1116–1125. Efficacy of infliximab after loss of response of/intolerance to adalimumab in pediatric Crohn’s disease: A retrospective multicenter cohort study of the “GETAID pédiatrique”
Key findings: In this retrospective study, 27 of 32 patients (84.4%) were still on IFX at 12 months of the switch. Among them, 13 had discontinued ADA because of a LOR, 12 for insufficient response and 2 due to primary nonresponse. At 1 year, 22 patients were in corticosteroid free clinical remission (68.7%).

PV Patel et al. JPGN 2024; 78:1126–1134. Real‐world effectiveness of ustekinumab and vedolizumab in TNF‐exposed pediatric patients with ulcerative colitis
Using the ICN registry, this observational study had 262 anti-TNF refractory patients receiving VDZ and 74 patients receiving UST. Key finding: At 6 months, 28.3% of patients on VDZ and 25.8% of those on UST achieved CFCR (p= 0.76)


X Liu et al. Gastroenterol 2024; 166:921-924. Machine Learning–Based Prediction of Pediatric Ulcerative Colitis Treatment Response Using Diagnostic Histopathology
M Iannucci et al. Gastroenterology 2024: 166: 730-732. Editorial. Open Access! A Baby Step or a Real Giant Stride: Histomic Enabled by Artificial Intelligence to Predict Treatment Response in Pediatric Patients With Ulcerative Colitis
In this article, their machine learning algorithm was trained on 187,571 informative patches from rectal hematoxylin and eosin biopsy samples from 292 treatment-naive pediatric patients with UC in a multicenter inception cohort (PROTECT) 2) study.
Key findings (summarized by editorial):
An important limitation on this study was that the population was 83% white, indicating that it may have less applicability in other cohorts.
My take (borrowed from editorial): Although this study focused solely on the therapeutic outcomes of mesalamine on treatment-naïve patients, it is anticipated that a comparable methodology (based on the fusion of machine learning and digital histopathology) could be applied in subsequent research to elucidate the necessity for colectomy, evaluate responses to biological agents, and optimize drug selection from the current armamentarium. In other words, this approach utilizing routine biopsy specimens may offer a pathway toward a precision personalized approach to managing pediatric-onset UC (and possibly IBD more broadly).

PS Dulai et al. Gastroenterol 2024; 166: 396-408. Open Access! Integrating Evidence to Guide Use of Biologics and Small Molecules for Inflammatory Bowel Diseases
“In this review, we provide a framework for clinicians and researchers to understand key differences in sources of evidence, how different methodologies are applied to study the comparative effectiveness of advanced medical therapies in IBD, and considerations for how these sources of evidence can be used to better integrate current guideline recommendations.”
This article explains the use of randomized controlled trials, “real-world evidence”/observational comparative studies, network meta-analysis, and post-hoc comparisons from randomized studies.
“The authors advocate for “”Given the rapidity with which new advanced medical therapies are becoming available in IBD, which quickly make current guidelines obsolete, living guidelines may offer a unique consideration to ensure applicability to routine care.”
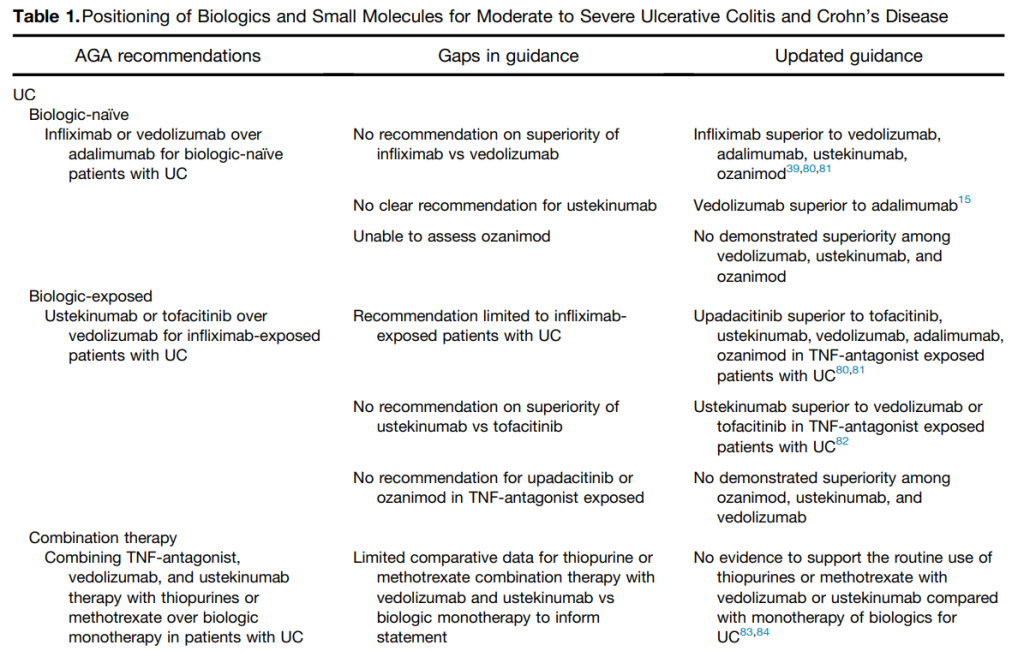

My take: This article provides a useful update of current advanced therapies and information in positioning these advanced therapies. It would be a great service if the IBD community could create something similar to HCVguidelines.org. The latter was a coordinated effort by the AASLD and IDSA to help provide expert advice during a deluge of amazing advances in HCV. And just like HCVguidelines, it is important to address “special” populations including pediatric patients and patients with very early onset IBD.
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
R Lujan, R Buchuk et al. Gastroenterol 2024; 166: 815-825.Early Initiation of Biologics and Disease Outcomes in Adults and Children With Inflammatory Bowel Diseases: Results From the Epidemiology Group of the Nationwide Israeli Inflammatory Bowel Disease Research Nucleus Cohort
Methods: All patients diagnosed with CD or UC in Israel (2005–2020) were included in the Epidemiology Group of the Israeli Inflammatory Bowel Disease Research Nucleus cohort. The authors compared disease duration at biologics initiation (ie, 0–3 months, >3–12 months, >1–2 years, and >2–3 years) using the cloning, censoring, and weighting by inverse probabilities method to emulate a target trial, adjusting for time-varying confounders and selection bias.
Of the 34,375 included patients (of whom 5240 [15%] were children), 7452 of 19,264 (39%) with CD and 2235 of 15,111 (15%) with UC received biologics. To attempt to adjust for patient characteristics, “essentially, each patient was cloned 4 times and 1 clone was assigend to each treatment strategy at baseline…[they were removed] subsequently if they did not receive the biologic within the acceptable time window.” Key findings:
Thus, overall, the study found that early initiation of biologics was associated only with a modest reduced risk of surgery and steroid dependency for Crohn’s disease. It was not found to reduce risk of colectomy or steroid dependency in UC.
My take: In my view, this study probably underestimates the benefits of early biologic therapy. Even though, lead time bias can skew interpretation, inadequately-treated disease likely leads to long-term damage. The associated editorial (pg 728) by Murphy notes that despite the sophisticated statistical methodology, all observational studies cannot fully control for unmeasured confounders.
Related blog posts:

This past weekend there were two fun events in Atlanta -Porchfest (Virginia Highlands) and Midtown Garden Stroll. Porchfest featured more than 50 local bands.
Here’s a couple photos from the Midtown Garden Stroll:





RS Dalal et al. Clin Gastroenterol Hepatol 2024; 22: 666-668. Comparative Effectiveness of Upadacitinib vs Ustekinumab for Ulcerative Colitis: A Multicenter Retrospective Cohort Study
This was a multicenter retrospective cohort study of adults with ulcerative colitis comparing upadacitinib (n=70) to ustekinumab (n=148). The upadacitinib-treated patients were all bio-exposed, had more advanced therapy failures, and higher baseline SCCAI (simple clinical colitis activity index).
Key findings:
My take: Upadacitinib had better response rates within 52 weeks even though the patients receiving this medication had more advanced therapy failures. However, it is important to keep in mind the limitations of this retrospective study. The improved outcomes are in contrast to a study comparing another JAK inhibitor (tofacitinib) to ustekinumab in which the outcomes appeared equivalent (Tofacitinib vs Ustekinumab -Which is Better for Ulcerative Colitis?).
Related blog posts:

Peyrin‐Biroulet, L., Arkkila, P., Armuzzi, A. et al. BMC Gastroenterol 24, 121 (2024). https://doi.org/10.1186/s12876-024-03163-5. Open Access! Comparative efficacy and safety of subcutaneous infliximab and vedolizumab in patients with Crohn’s disease and ulcerative colitis included in randomised controlled trials.
Methods: Studies included in the current analysis were parallel-group, randomised controlled trials (RCTs) that evaluated treatment with IFX SC, following induction therapy with IFX IV, or treatment with VDZ (either with VDZ IV or with VDZ SC [following IV induction therapy]). The authors identified three eligible CD trials and four eligible UC trials that assigned over 1200 participants per disease cohort to either IFX SC or VDZ.
Key findings:
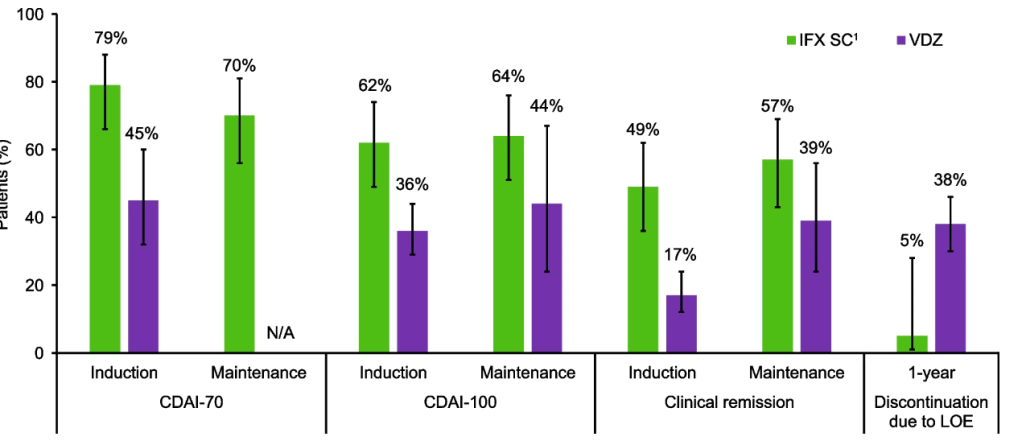
Crohn’s disease: Intravenous induction therapy with IFX demonstrated better efficacy compared with VDZ; during the maintenance phase, IFX SC showed numerically better efficacy than VDZ. A lower proportion of IFX SC-treated patients discontinued therapy due to lack of efficacy over 1 year.
Ulcerative colitis: Efficacy profiles were similar with IFX SC and VDZ during the induction and maintenance phases, and a lower proportion of IFX SC-treated patients discontinued therapy due to lack of efficacy over 1 year.
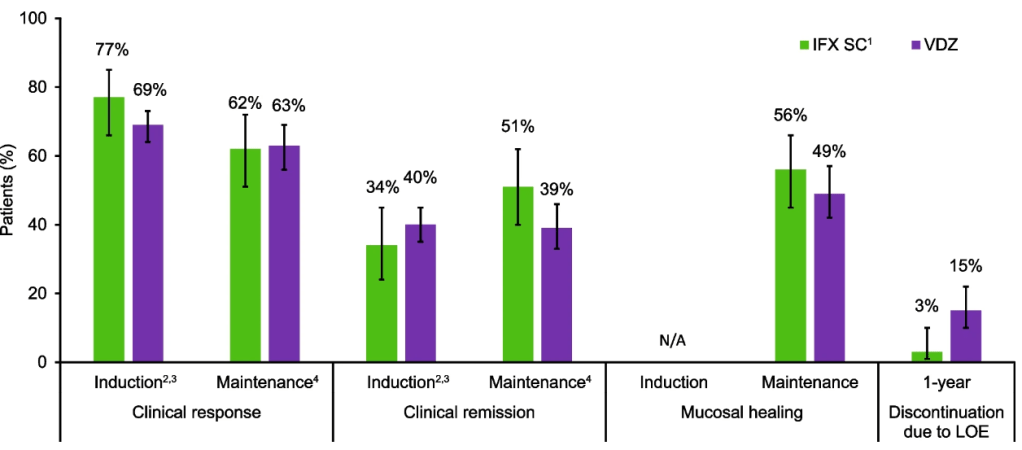
Safety: In both cohorts, safety profiles for IFX SC and VDZ were generally comparable during 1 year.
Discussion Points:
My take: This study indicates that SC infliximab (like IV infliximab) appears to be more effective than vedolizumab for patients with Crohn’s disease and similarly effective for ulcerative colitis, keeping in mind the aforementioned discussion points. While not evident in this study, vedolizumab has a superior safety profile.
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
Thanks to Mike Hart for this reference.
V Sinopoulou et al. Cochrane Database of Systematic Reviews 2024 March 20. Prebiotics for induction and maintenance of remission in ulcerative colitis.
This study included 9 RCTs involving a total of 445 participants. Key findings:
My take: Currently, there is no solid evidence to recommend prebiotics in patients with ulcerative colitis.
Related blog posts:


K Mullin, RM Rentea, M Appleby, PT Reeves. Pediatrics in Review 2024; 45: 210-224. Gastrointestinal Ostomies in Children: A Primer for the Pediatrician
Like yesterday’s article on GTs, this is another terrific review with plenty of helpful images and advice regarding ostomy management.



My take: This is a very useful resource. Even a quick read will make clinicians appreciative of having the assistance wound ostomy nurses.
Related blog posts:
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.