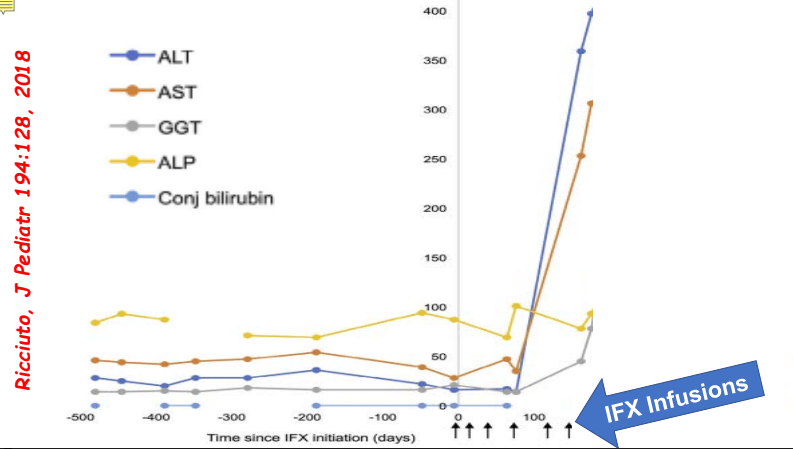
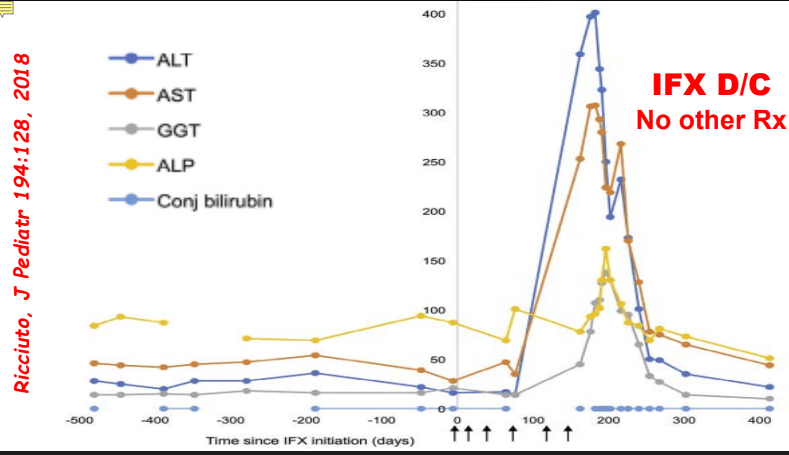
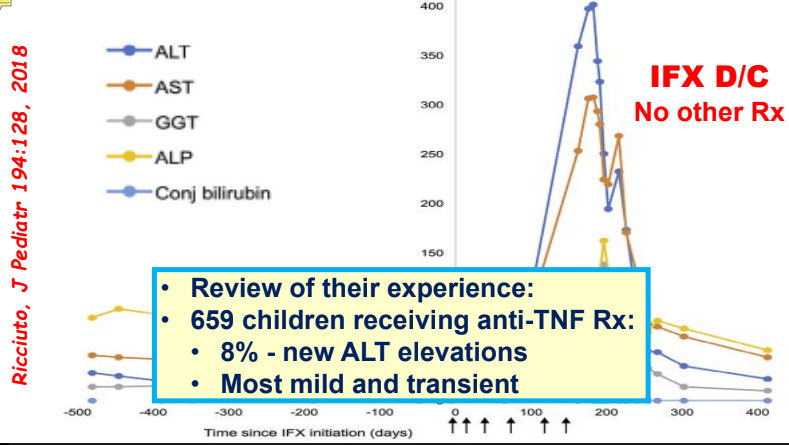
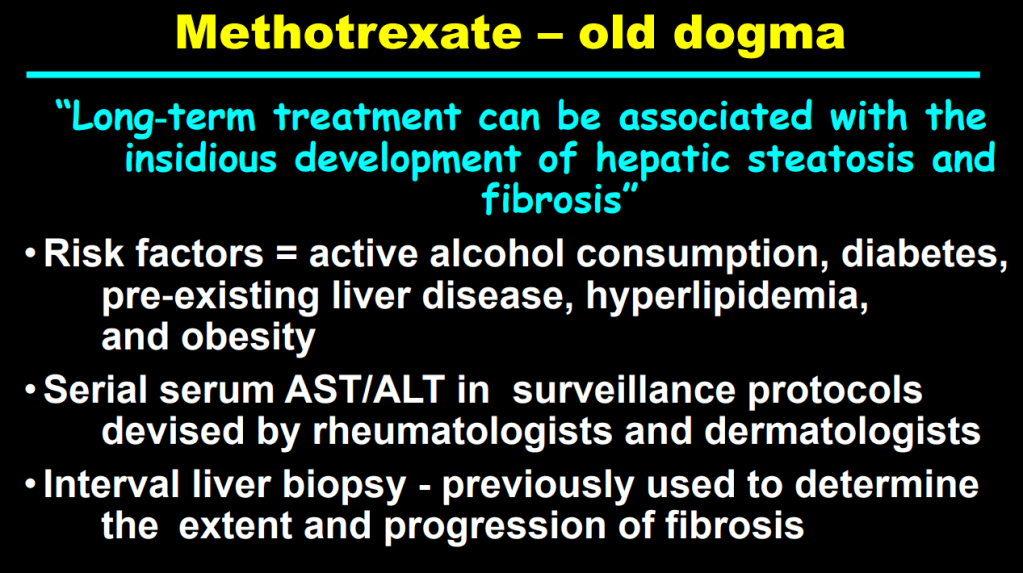
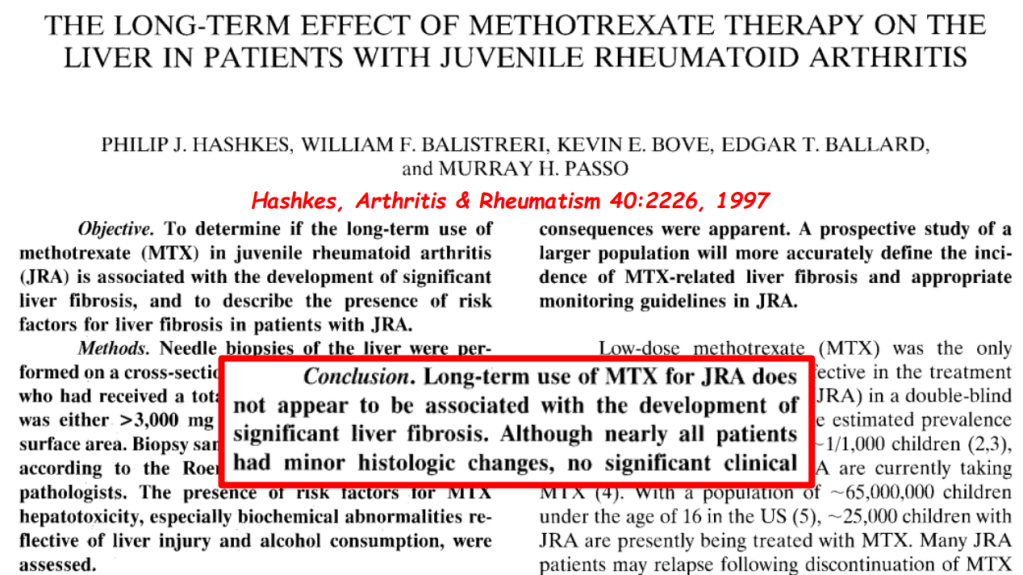
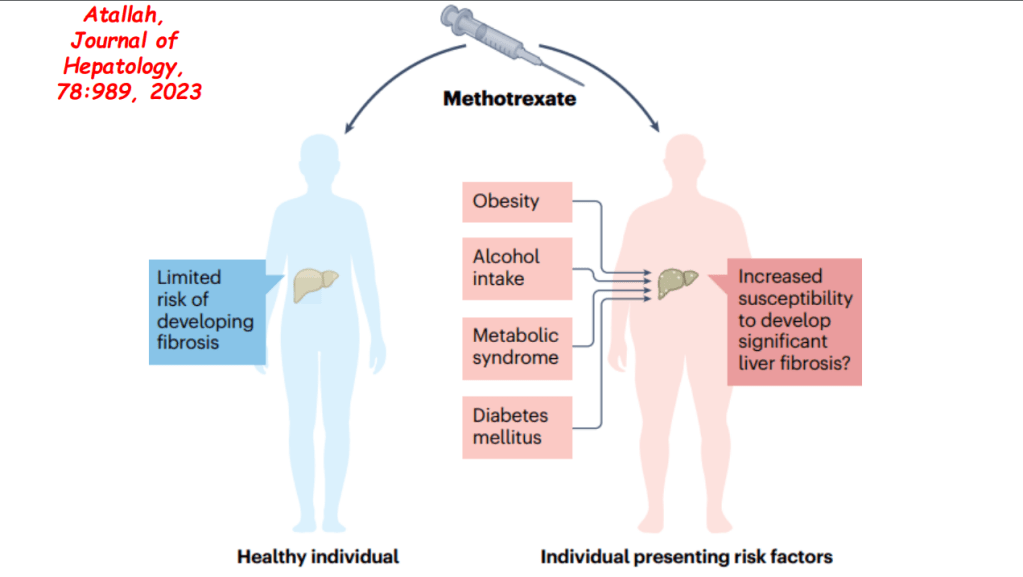
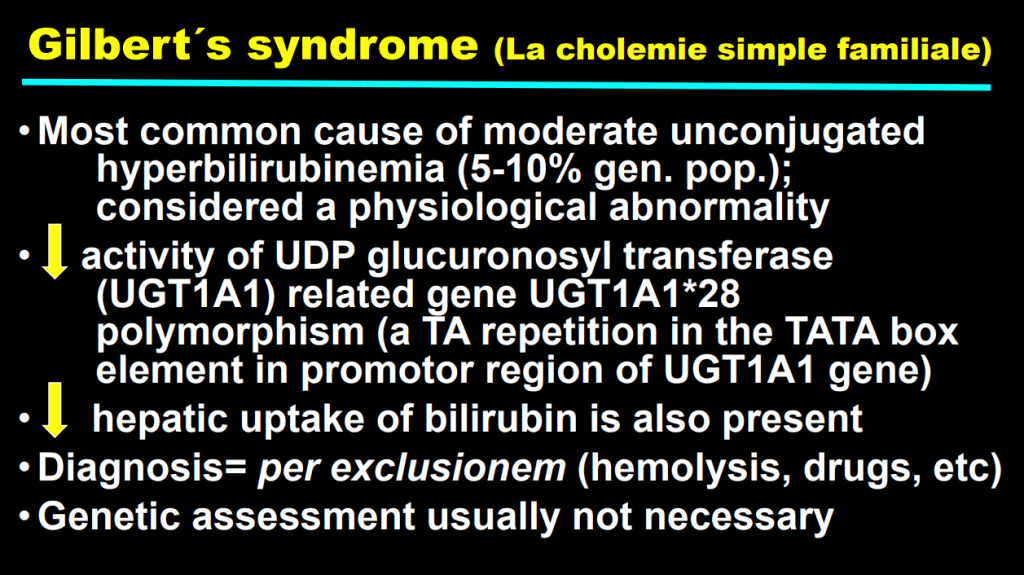
- F Koutny et al. JPGN 2024; https://doi.org/10.1002/jpn3.12194. Open Access! Poorly controlled pediatric type 1 diabetes mellitus is a risk factor for metabolic dysfunction associated steatotic liver disease (MASLD): An observational study
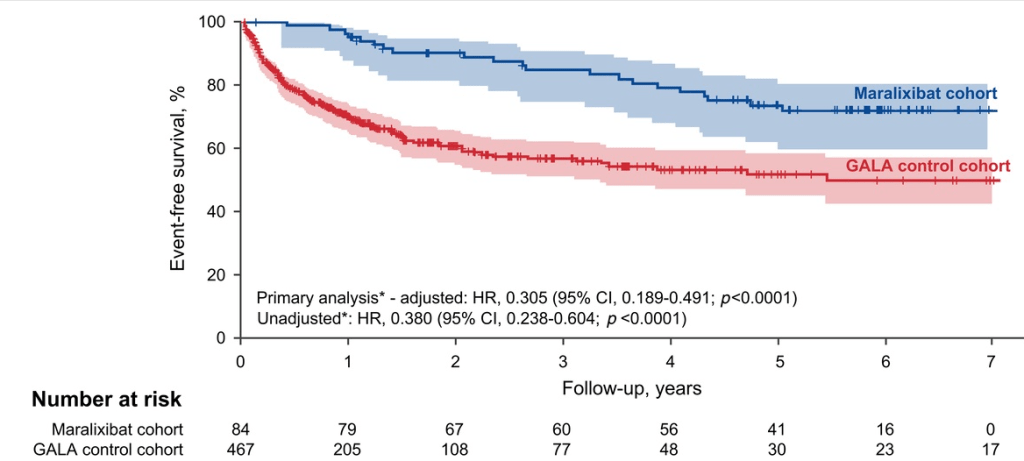
Study population, n=32,325. Key finding: Inadequately controlled T1D (HgbA1c >11%) was associated with a higher hazard ratio ((HR: 1.54) of elevated ALT values compared to children with controlled T1D over an observation period extending up to 5.5 years. When both elevated HbA1c (>11%) and overweight were present, the HR was 2.71.
————————————————————————————————————————–
2. G Indolfi et al. JPGN 2024; 78:957–972. ESPGHAN recommendations on treatment of chronic hepatitis C virus infection in adolescents and children including those living in resource-limited settings
Summary of Recommendations:

————————————————————————————————————————–
3. F Lacaille et al. JPGN 2024; 78:1374–1382. Awareness, referral and age at Kasai surgery for biliary atresia in Europe: A survey of the Quality-of-Care Task Force of ESPGHAN
Key finding: Data from 785 infants diagnosed with BA from 2015 to 2019 from 18 centers in 15 countries revealed a mean age at referral to tertiary center of 55 days (similar to results obtained in Europe 10–30 years earlier)
Related blog posts:
Biliary Atresia
- Don’t Put the Cart Before the Horse: Biliary Atresia Screening
- Why Didn’t Screening for Biliary Atresia Improve Outcome In This Study?
HCV:
- Why CDC is Drafting New Guidelines for Screening Children for Perinatally-Acquired Hepatitis C Infection
- NASPGHAN Foundation: Hepatitis C in Children and Adolescents
- Eradicating Hepatitis C Dependent on Congressional Action
- Hepatitis C is Undertreated in the U.S.
- Medical Progress: Toward Hepatitis C Elimination