FI Scott et al. Gastroenterology, Volume 169, Issue 7, 1397 – 1448; Open Access! AGA Living Clinical Practice Guideline on the Pharmacologic Management of Moderate-to-Severe Crohn’s Disease
The guideline panel agreed on 16 recommendations. This highly-detailed report provides a comprehensive, patient-centered, evidence-based approach to the pharmacologic management of adult patients with moderate-to-severely active CD. Table 1 summarizes this lengthy 53-page report. Tomorrow’s post will be the “spotlight” summary which presents the recommendations in easier to read graphic.

Key Points:

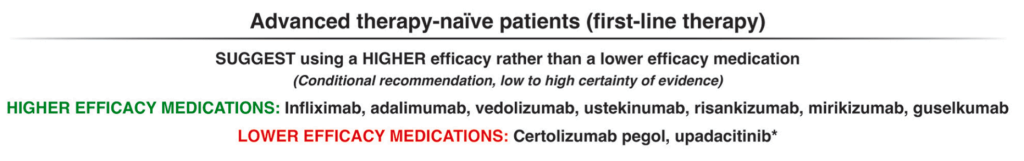

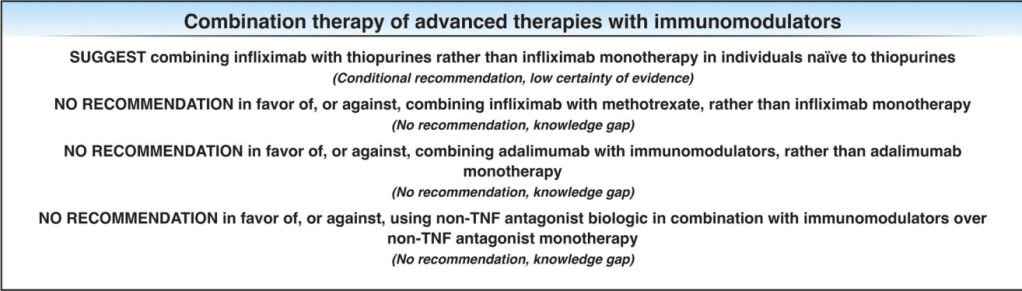
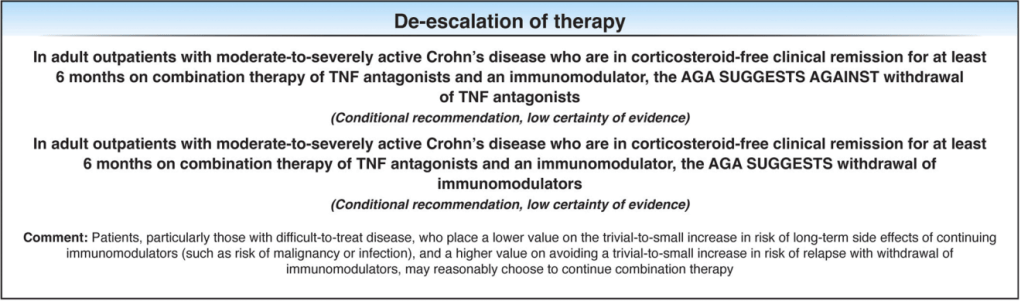
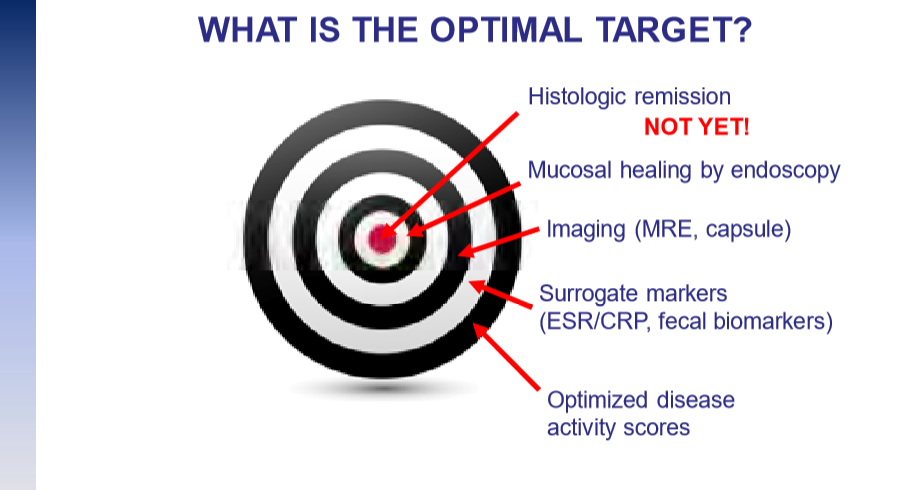
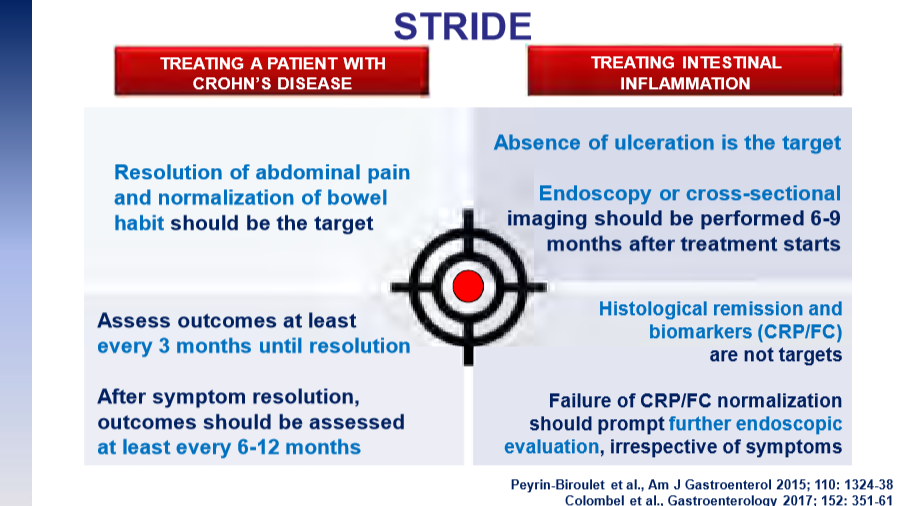
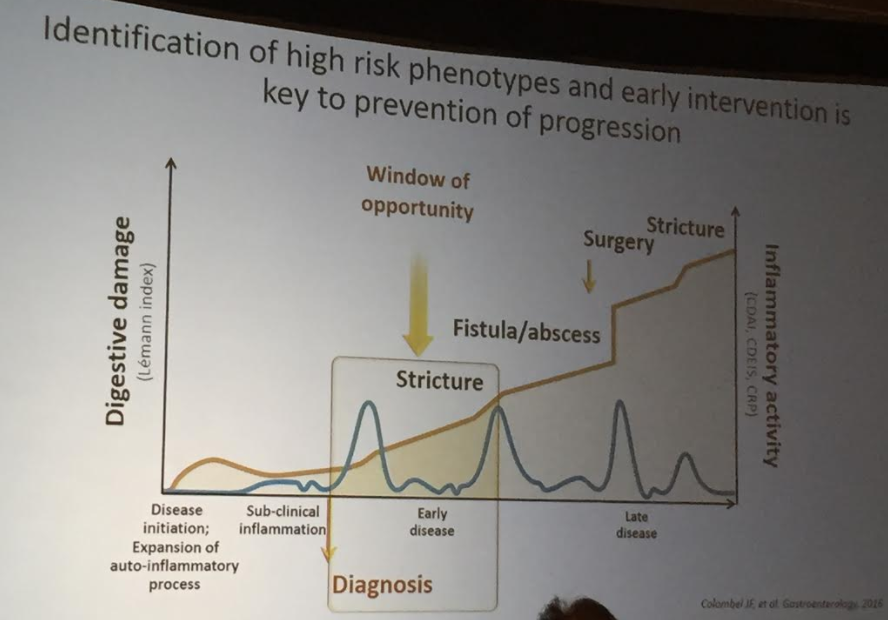
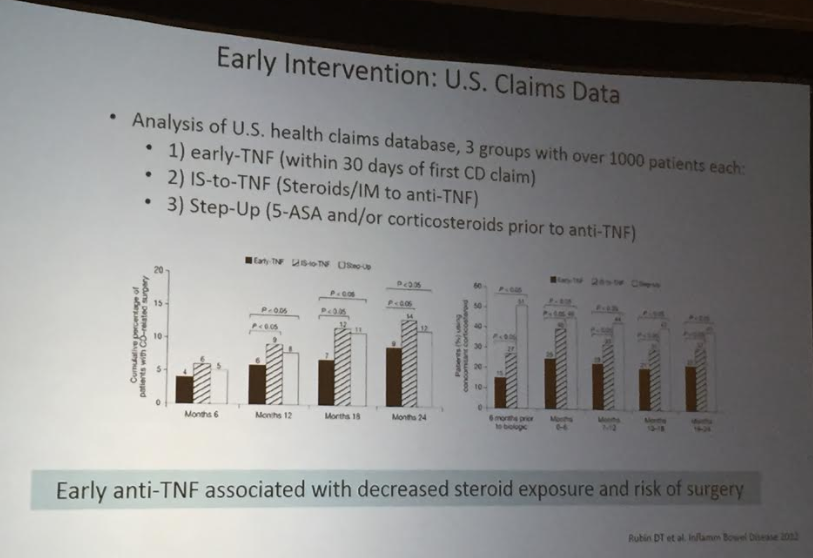
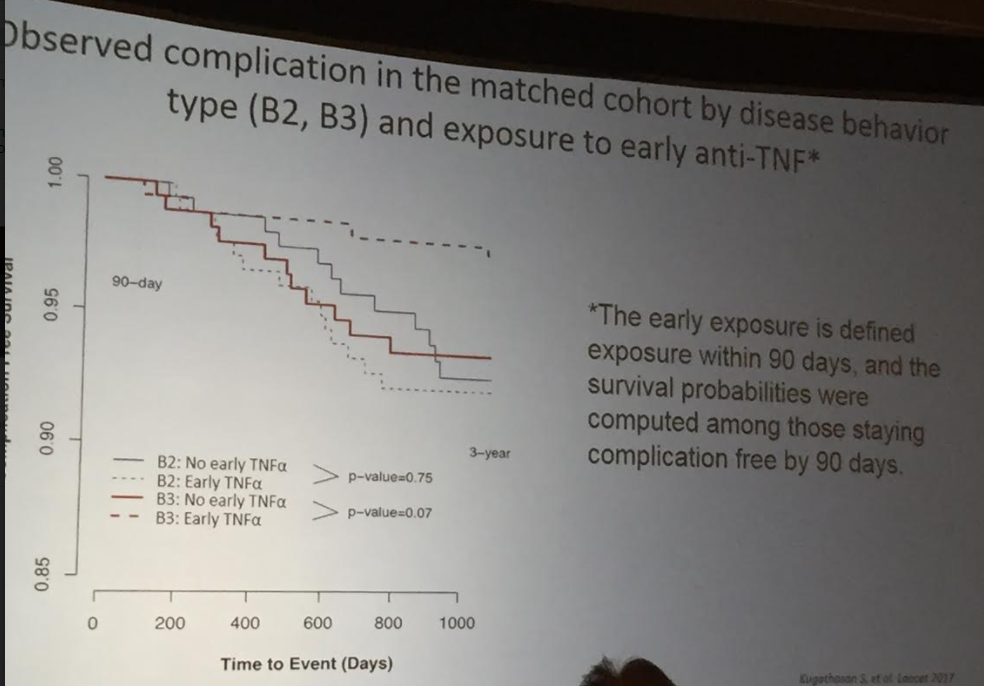
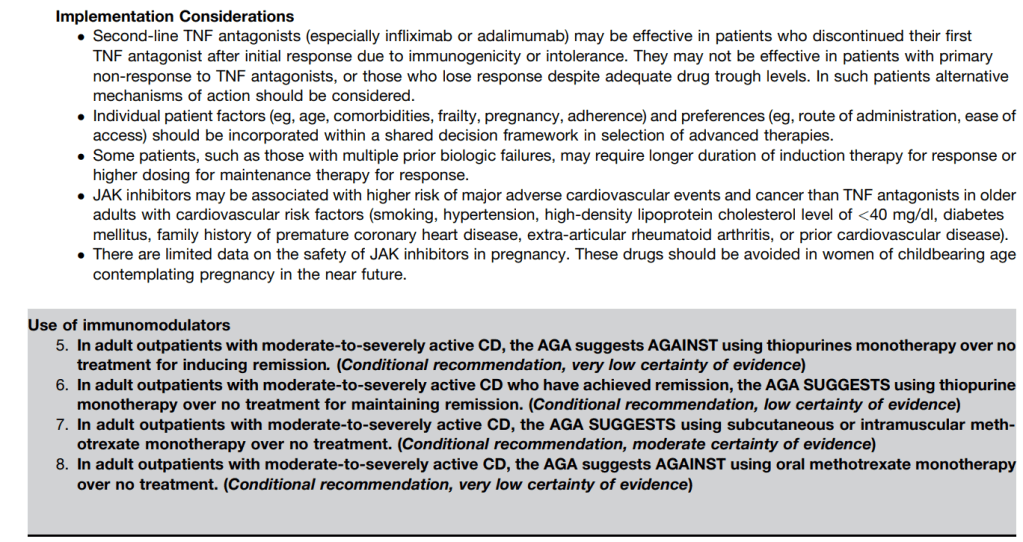
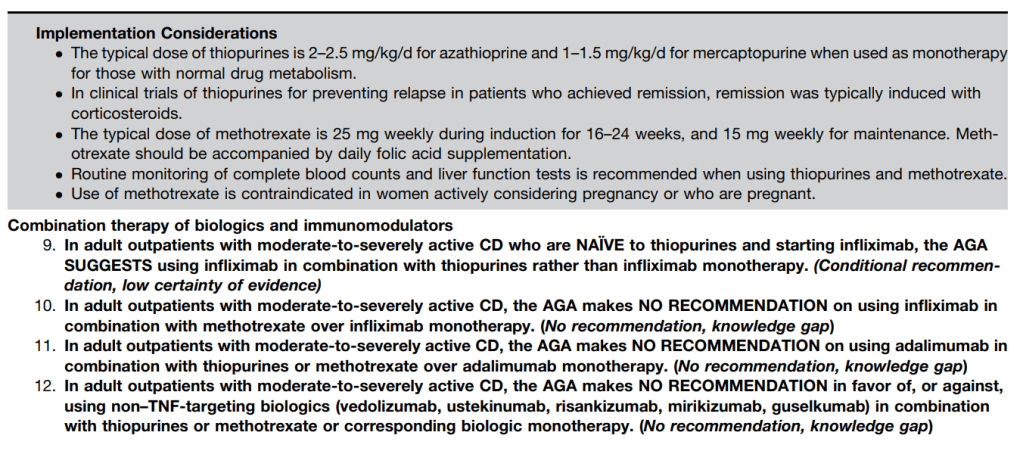
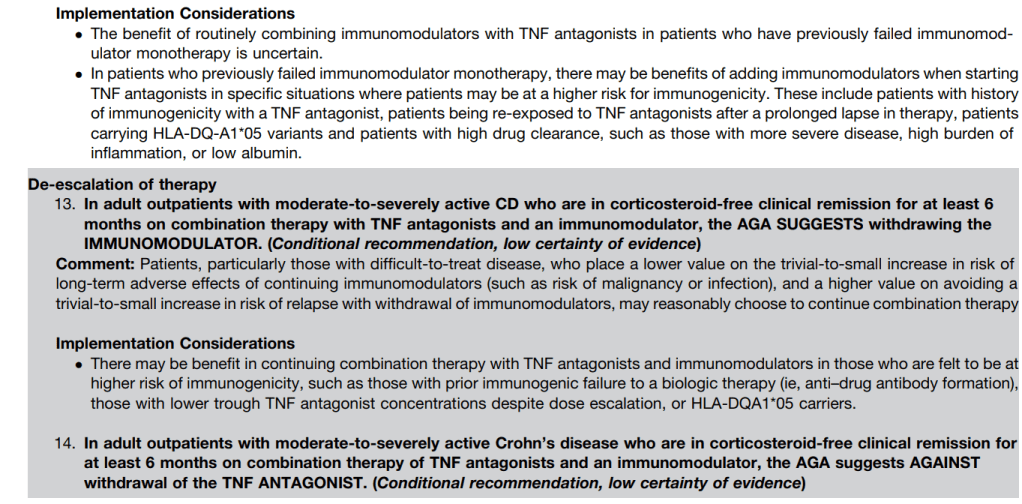
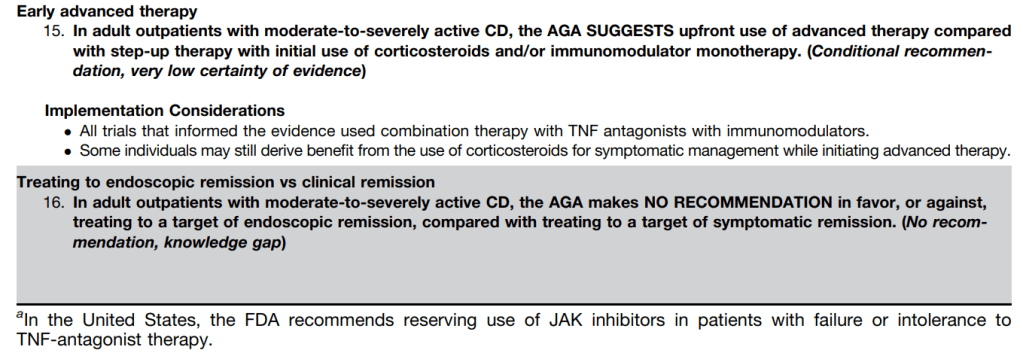
The guidelines are overall very helpful. They identify higher efficacy medications and recommend them. In addition, they support the use of combination therapy with thiopurines (which are less frequently used in pediatrics). It is interesting that the sixteenth recommendation clashes with prior expert recommendations. The sixteenth recommendation in this report makes no recommendation on using endoscopic surveillance compared to symptomatic clinical remission. Most experts advise “treat-to-target” therapy approaches.
In the discussion of this, the authors state the followiing:
“Recent position statements from an international consortium of experts have advised that longitudinal targets for the management of IBD should include not only clinical remission but also endoscopic resolution of inflammation.31 Several studies have demonstrated that patients who achieve endoscopic remission (vs those with ongoing endoscopic activity) have favorable long-term outcomes…
There are limited RCTs assessing whether there is actual benefit in systematically treating toward endoscopic remission target vs symptomatic remission targets (ie, testing whether the target has been achieved, followed by algorithmic treatment adjustment, including escalating index therapy, adding an immunomodulator, followed by switching to an alternative advanced therapy and surgery). There was significant heterogeneity among the 2 reviewed studies, both in terms of the advanced therapy used, algorithms for therapy modification, and the cadence and frequency of endoscopic monitoring that challenge interpretation. Based on the significant uncertainty of evidence with regard to improving maintenance of remission or reducing the risks of adverse events, the guideline panel could not make a recommendation in relation to selecting endoscopic targets over clinical targets.
It is worth emphasizing that in both of the included trials, the majority of individuals in the endoscopic healing arms were not able to meet the goal of endoscopic healing despite an algorithmic approach. For example, in STARDUST, only 11% of individuals achieved endoscopic remission.149…There are specific patient populations, such as those who have recently undergone intestinal resection,155 in which endoscopic evaluation may be particularly valuable in clinical decision making...
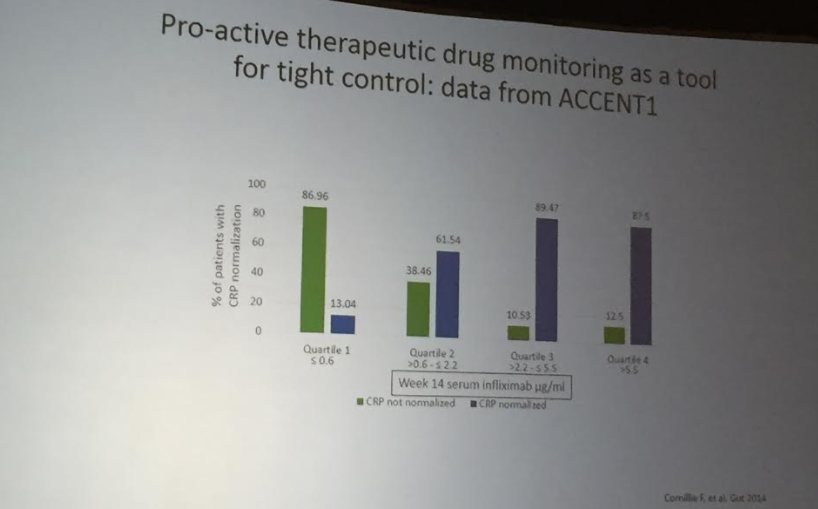
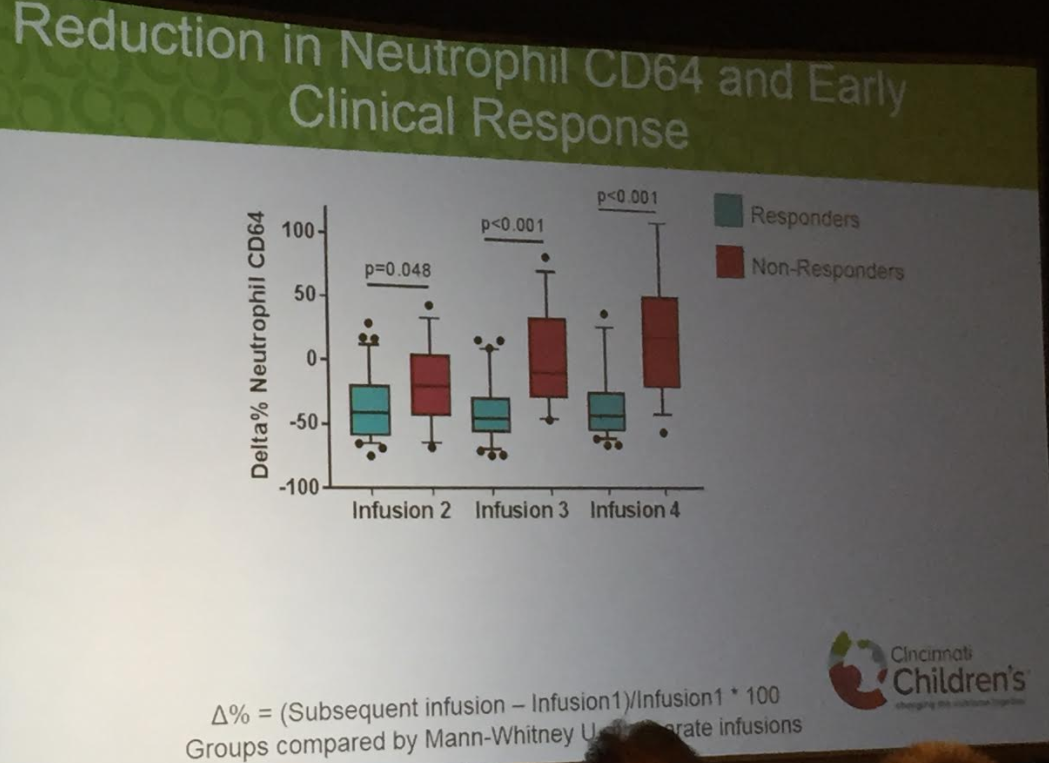
The benefit of a monitoring strategy incorporating biochemical monitoring over clinical monitoring alone was demonstrated in the CALM trial,152 and has been addressed in previous AGA guidelines on the role of biomarkers in patients with CD.12“
My take: These “living” guidelines are likely to be quite influential in selecting Crohn’s disease therapy. In pediatrics, ImproveCareNow provides a similar role of guiding treatment.
Related blog posts:
Guidelines for UC:
- AGA Living Guideline for Moderate-to-Severe Ulcerative Colitis –The Good and The Bad
- Pediatric Guidelines for Ulcerative Colitis (Part 1)
- Pediatric Guidelines for Ulcerative Colitis (Part 2: Acute Severe Colitis)
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis (2024)
Crohn’s Disease:
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- Comprehensive ACG Clinical Guidelines for Crohn’s Disease (2025)
- “A Practical Guide to Diet and IBD” (2025)
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- Is It RISKy Not To Use Anti-TNF Therapy for Pediatric Crohn’s Disease?