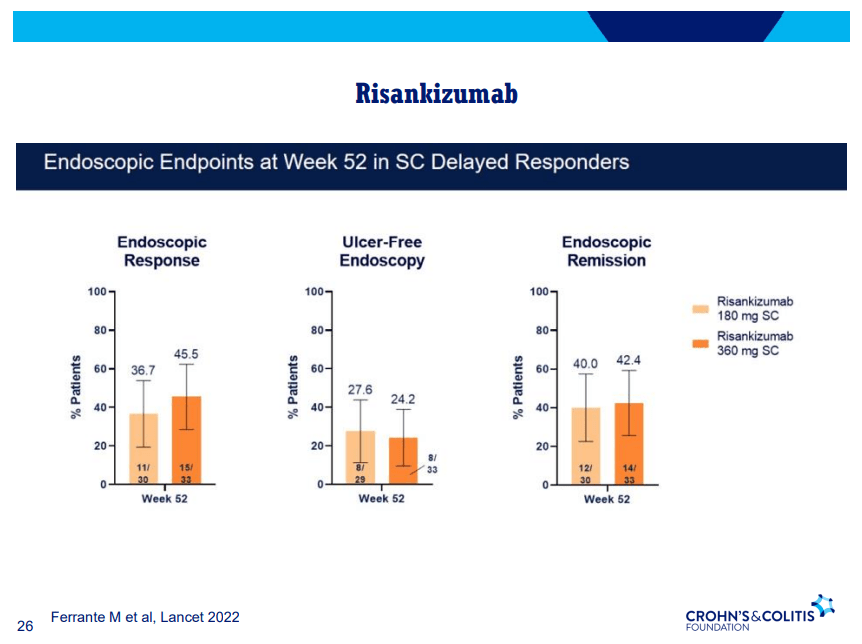
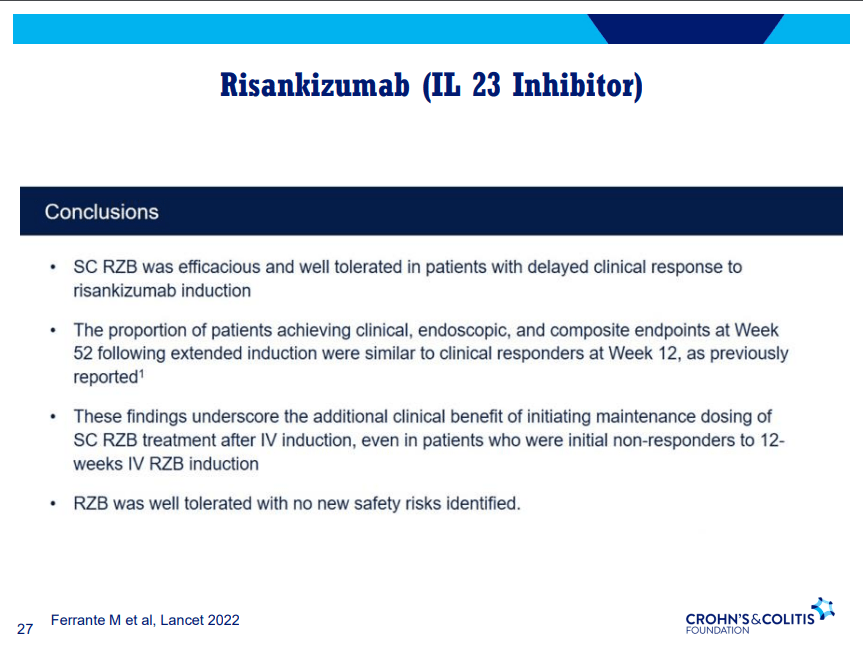
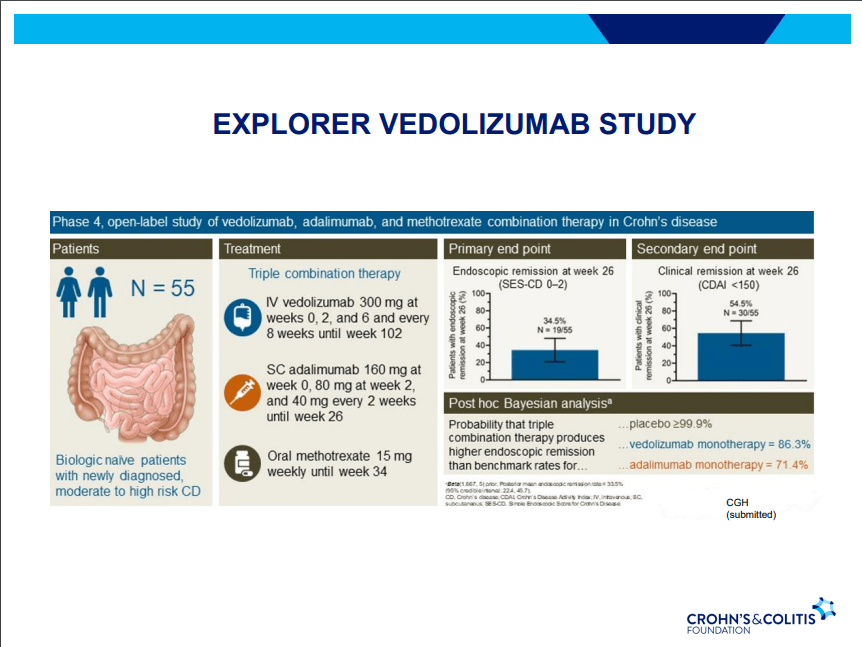
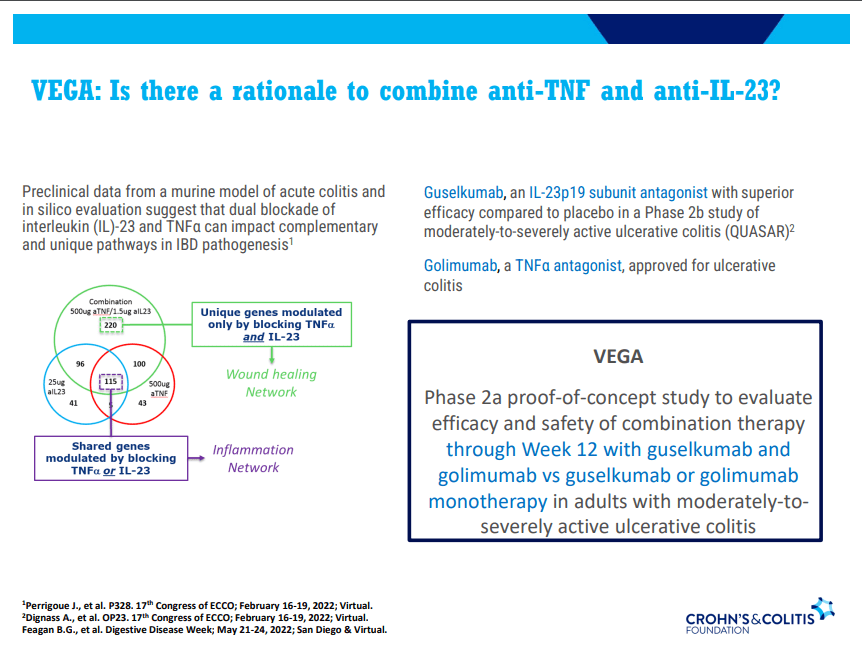
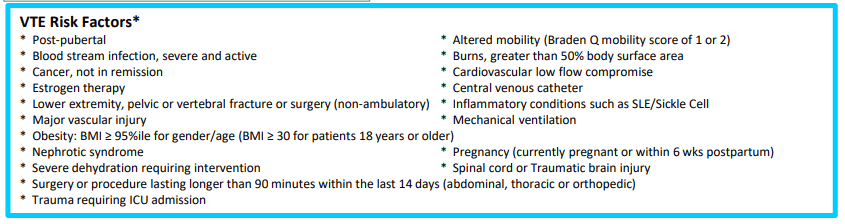
A Yu et al. Clin Gastroenterol Hepatol 2023; 21: 3115-3124. Open Access! Real-World Experience With Tofacitinib Dose De-Escalation in Patients With Moderate and Severe Ulcerative Colitis
On May 30, 2018, the US Food and Drug Administration (FDA) expanded the indication of tofacitinib (Xeljanz; Pfizer), an oral Janus kinase (JAK) inhibitor, for the treatment of adults with moderately to severely active ulcerative colitis. However, the optimal dosing remains unclear.
In this “real-world” study by Yu et al, a retrospective review of 162 patients was conducted (2012-2022). 52% continued 10 mg twice daily while 48% underwent dose de-escalation to 5 mg twice daily. The primary outcome was evidence of UC disease activity–related events: hospitalization/surgery, corticosteroid initiation, tofacitinib dose increase, or therapy switch.
Key findings:
- Cumulative incidence rates of UC events at 12 months were similar in patients with and without dose de-escalation (56% vs 58%; P = .81)
- An induction course with 10 mg twice daily for more than 16 weeks was protective of UC events (hazard ratio [HR], 0.37) while ongoing severe disease (Mayo 3) was associated with UC events (HR, 6.41)
- Twenty-nine percent of patients with UC events had their dose re-escalated to 10 mg twice daily, with only 63% able to recapture clinical response at 12 months
Discussion Points:
- “Although the product label recommends dose de-escalation after 8 or 16 weeks, clinical practice is variable in the real-world setting… In this retrospective real-world study of moderate to severe UC patients with almost half undergoing dose de-escalation, we observed that more than half of patients experienced a UC disease activity–related event within 12 months after dose de-escalation, particularly in patients with an induction course of fewer than 16 weeks and active endoscopic disease at 6 months after induction…”
- ” Although dose de-escalation is preferable for long-term maintenance therapy to reduce the potential lifetime risk of medication-related adverse events [eg. VTE], it must be balanced with sustained remission to prevent short- and long-term disease-related complications.”
- “In the OCTAVE study which reported higher rates of long-term remission, patients de-escalated only after having shown clinical and endoscopic remission after 52 weeks on tofacitinib 10 mg twice daily”
My take (borrowed from authors): “Emphasis should be placed on clinical and endoscopic evidence of improvement before consideration of dose de-escalation to ensure the highest probability of treatment success.” This advice, though, may conflict with product labelling which states that “tofacitinib induction with 10 mg twice daily beyond 16 weeks is not recommended; in fact, it is recommended to stop after 16 weeks if adequate response has not been achieved.”
Related blog posts:
- Tofacitinib Outperformed Vedolizumab in Anti-TNF-experienced Ulcerative Colitis
- Treatments for “Bad” Inflammatory Bowel Disease (Part 2) & Reassuring Data on Tofacitinib
- IBD Updates: Probability of Needing a Stoma with Crohn’s Disease, “CEASE” anti-TNF study, Extending Tofacitinib Response Time
- Increased Risk, Increased Reward (possibly) with Tofacitinib
- IBD Shorts: Tofacitinib Safety, Vit D post-op, EIM with Vedolizumab
- A New FDA Warning for Tofacitinib
- “Tofacitinib: A Jak of All Trades”
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.