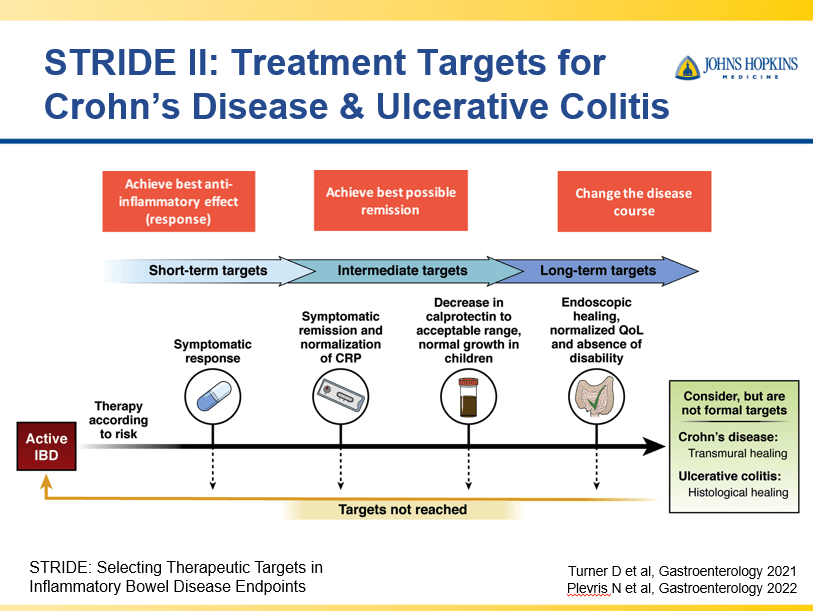
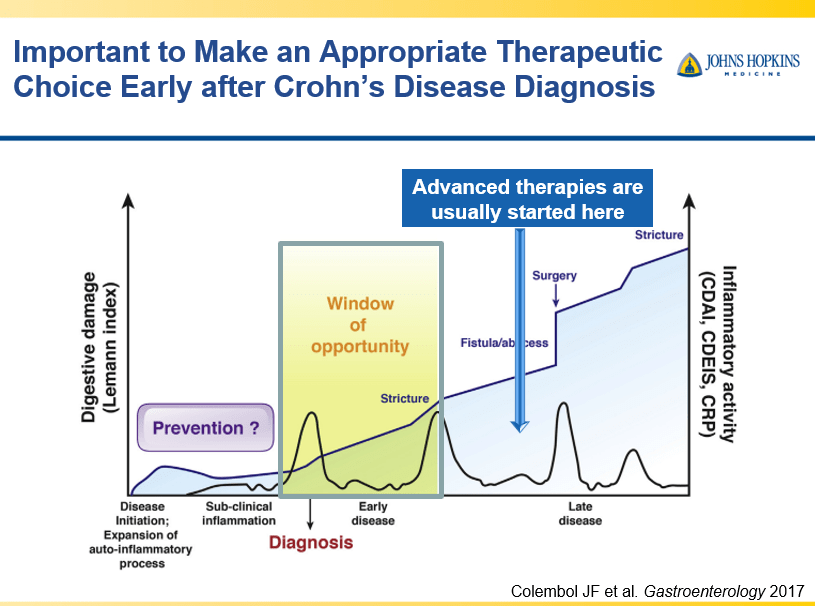
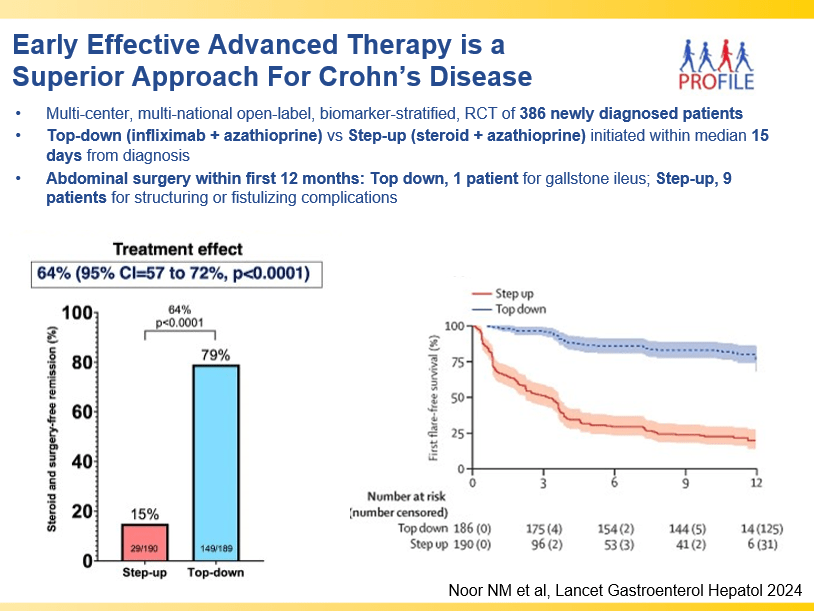
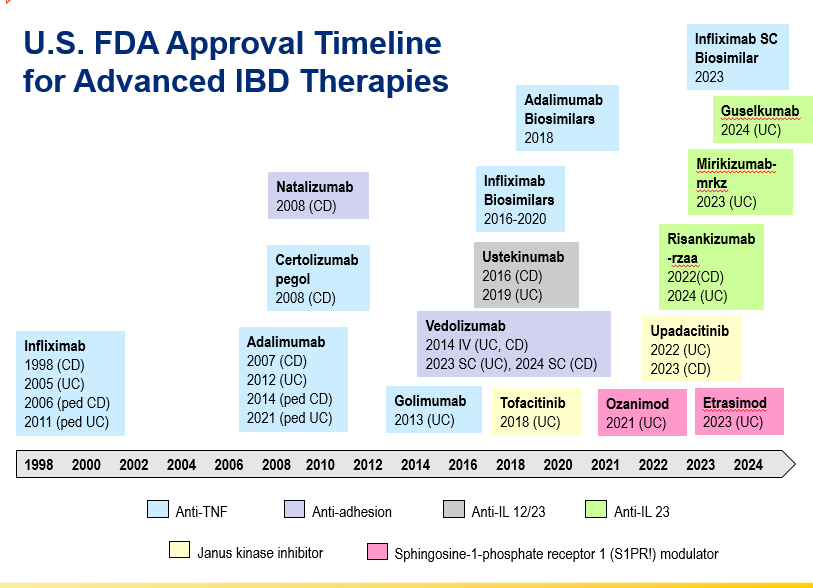
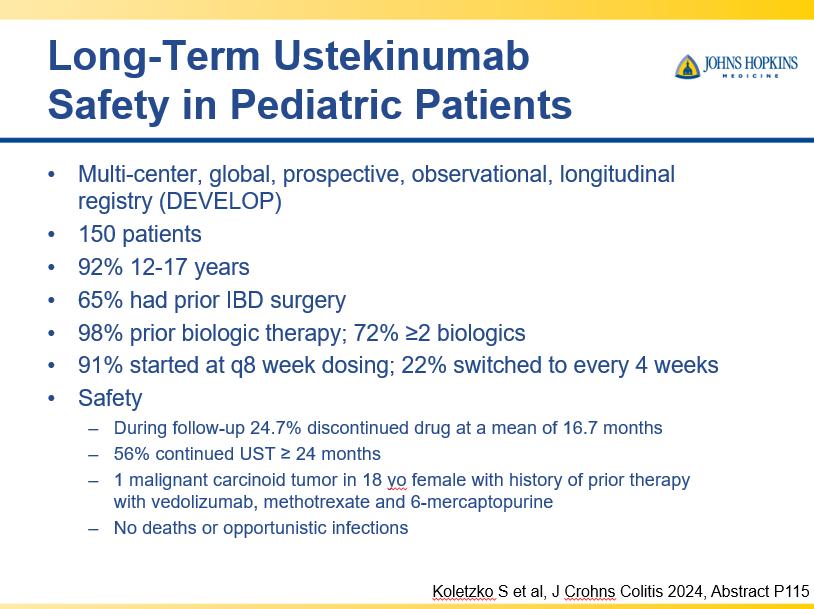
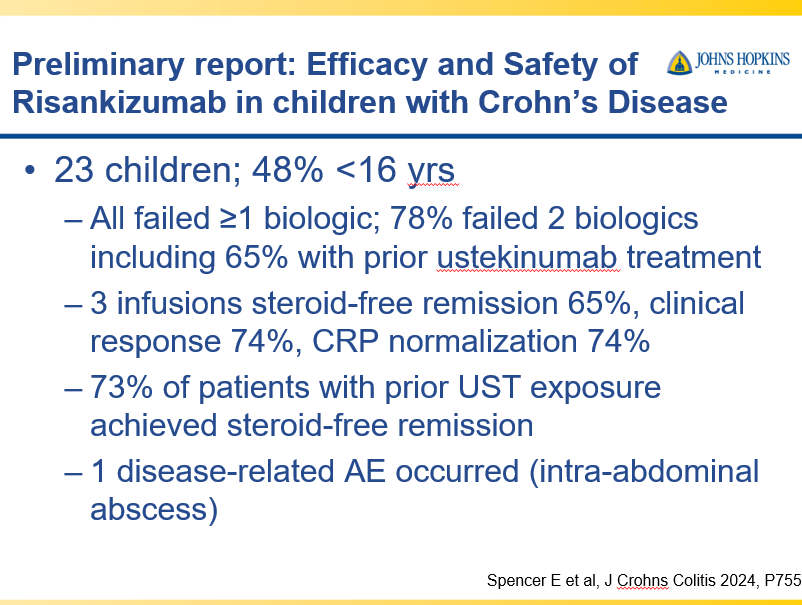
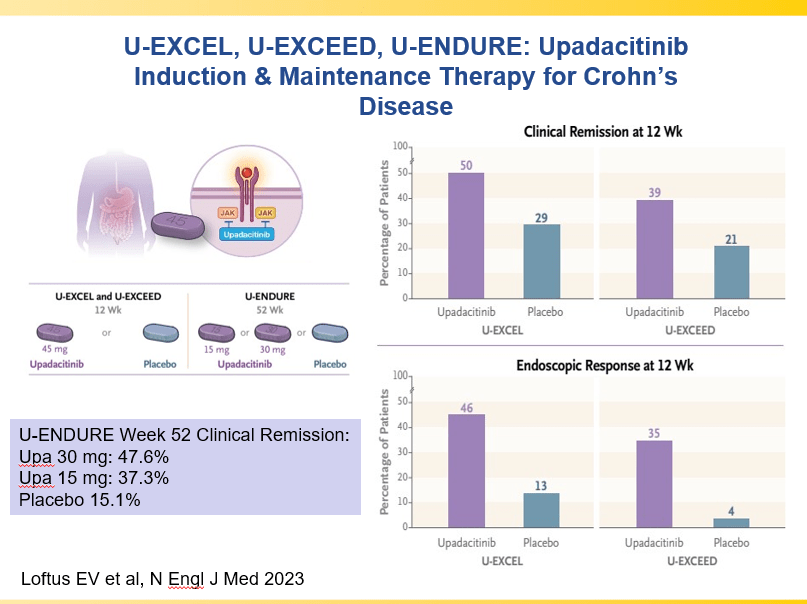
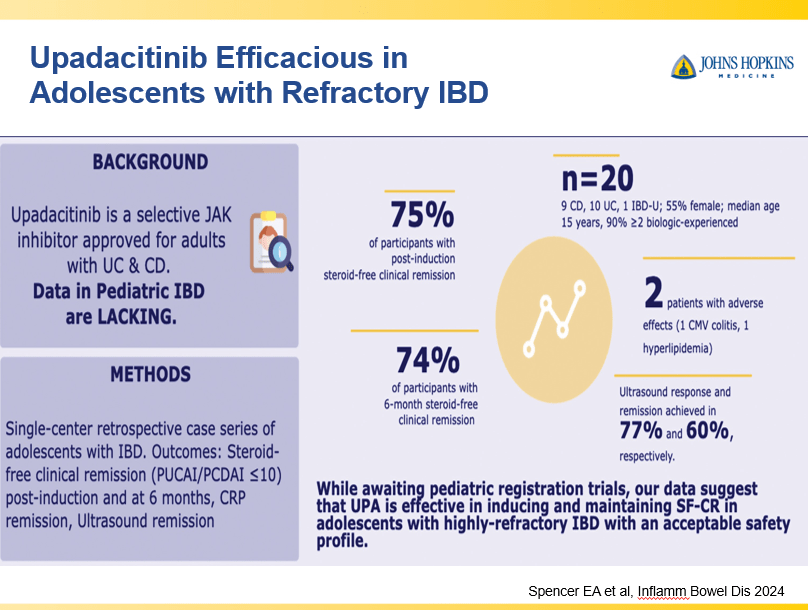
D Burnett et al. JPGN Reports. 2025;6:19–26. A Canadian multicenter pediatric eosinophilic esophagitis cohort: Evidence for a nondilation approach to esophageal narrowing
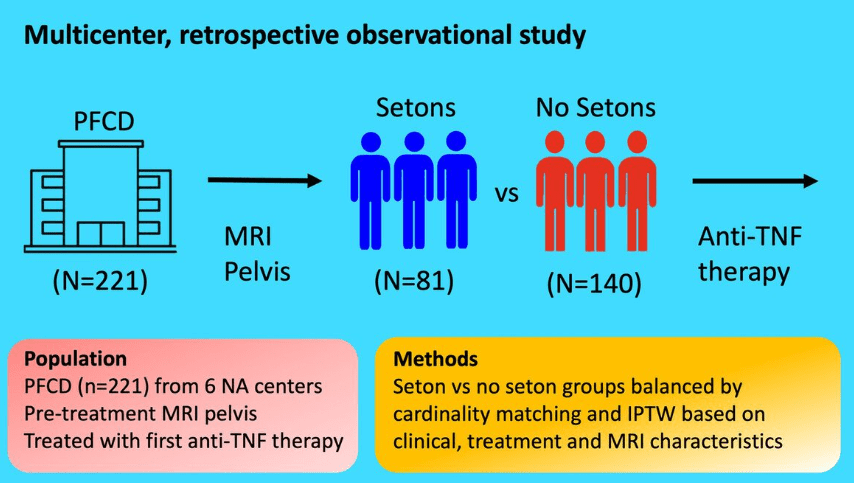
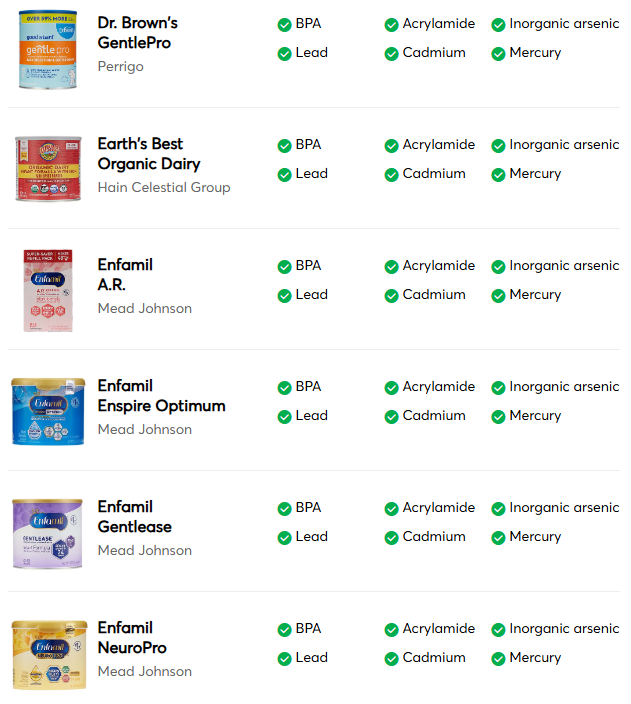
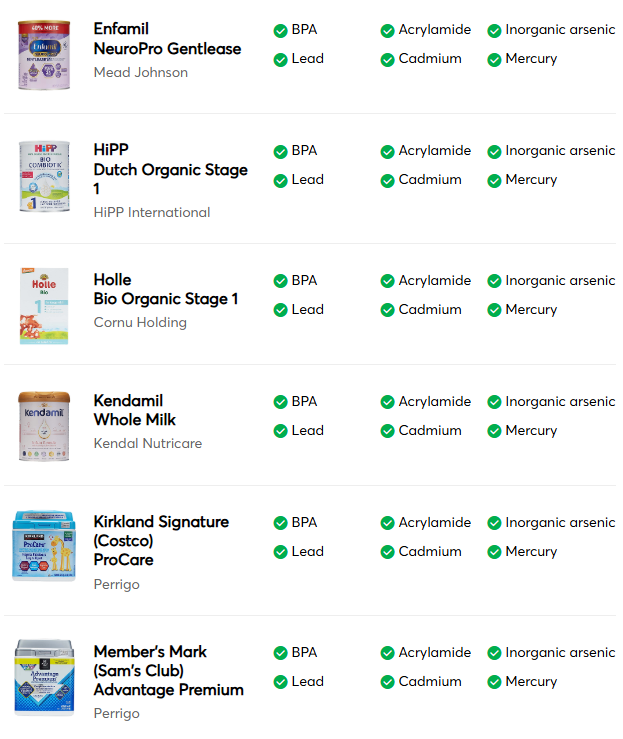
This was a retrospective study from Vancouver with 332 new diagnosis of eosinophilic esophagitis (EoE).
Key findings:
- The incidence of EoE in patients less than 15 years old was 5.4 per 100,000 person‐years
- Of the 332 new diagnoses, 40 (12.0%) had endoscopically-identified esophageal narrowing at diagnosis
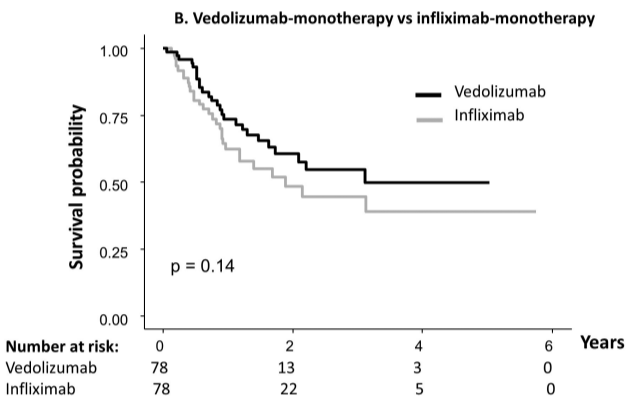
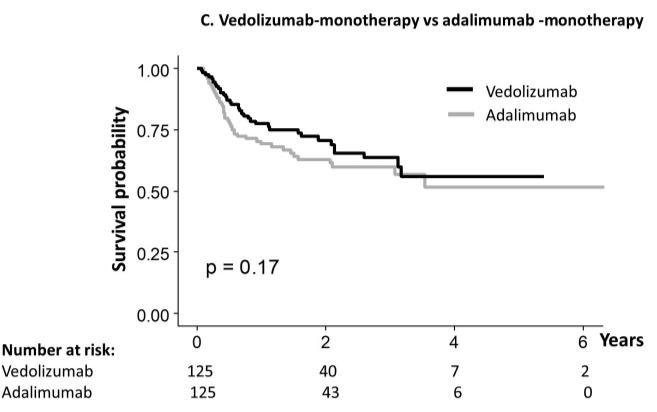
- During follow-up of 1-4 years, 11 (27.5% of narrowed cohort) patients underwent mechanical esophageal dilation
- “Our most surprising result was the high number of cases of esophageal narrowing that resolved on follow‐up scope after initiating medical/dietary therapy, without need for mechanical dilation.” The rates of resolution were 1 with 1 (100%) on systemic steroids, 7 out of 13 (54%) with topical steroids, 3 out of 4 (75%) with dietary therapy, and 4 out of 12 (33%) with PPI therapy
My take: This study was a little confusing in how the results are presented. Through most of the article, there are 40 (of 332) children with newly-diagnosed EoE who had narrowing identified. However, there is also discussion of a 65 children subset who had follow-up endoscopy and this is the group in which esophageal narrowing treatment response is reported.
Despite the confusion, the clear take-home message is that esophageal narrowing often responds to medical treatment; only a subset of children with esophageal strictures need mechanical dilatation.
Related blog posts:
- Frequency of Strictures in Pediatric Eosinophilic Esophagitis
- Practical Tips for Eosinophilic Esophagitis (Glenn Furuta,MD)
- Injecting Steroids for esophageal strictures -Does it work?
- “Esophageal Hypervigilance” and Outcomes in Eosinophilic Esophagitis
- Big Study on Intralesional Steroid Injection for Esophageal Anastomotic Strictures & 8 Truths on COVID-19 (March 2020)
- Evidence-Based Algorithm for Surveillance in Esophageal Atresia Patients