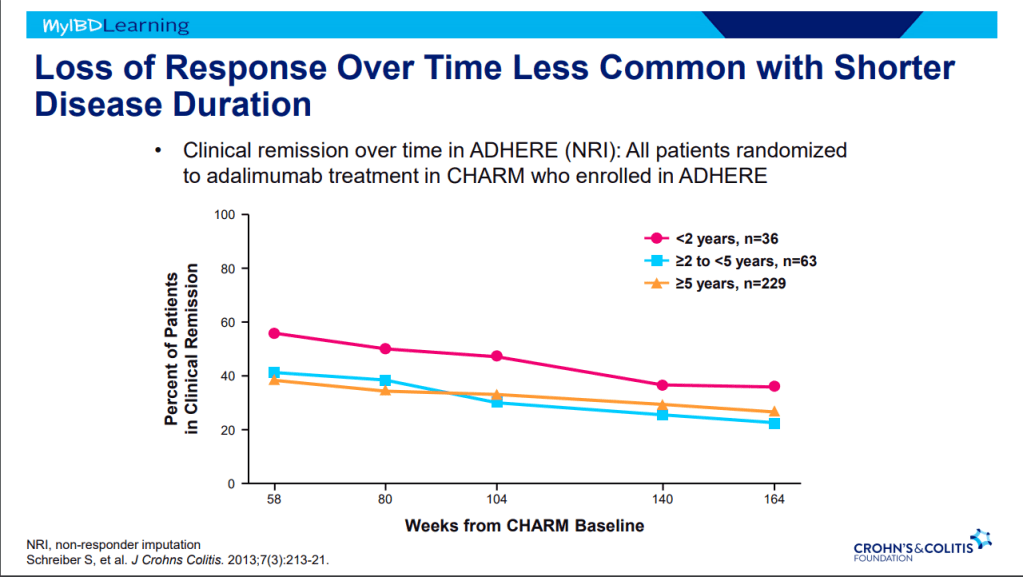
- G D’Haens et al. Inflamm Bowel Dis 2024; https://doi.org/10.1093/ibd/izae004. Extended Induction and Prognostic Indicators of Response in Patients Treated with Mirikizumab with Moderately to Severely Active Ulcerative Colitis in the LUCENT Trials
Key findings:
- Of patients not achieving clinical response during 12-week induction, 53.7% achieved response following extended induction (additional 3 doses of IV infusion every 4 weeks)
- With “extended induction,” total of 80.3% mirikizumab-treated patients achieved clinical response by W24
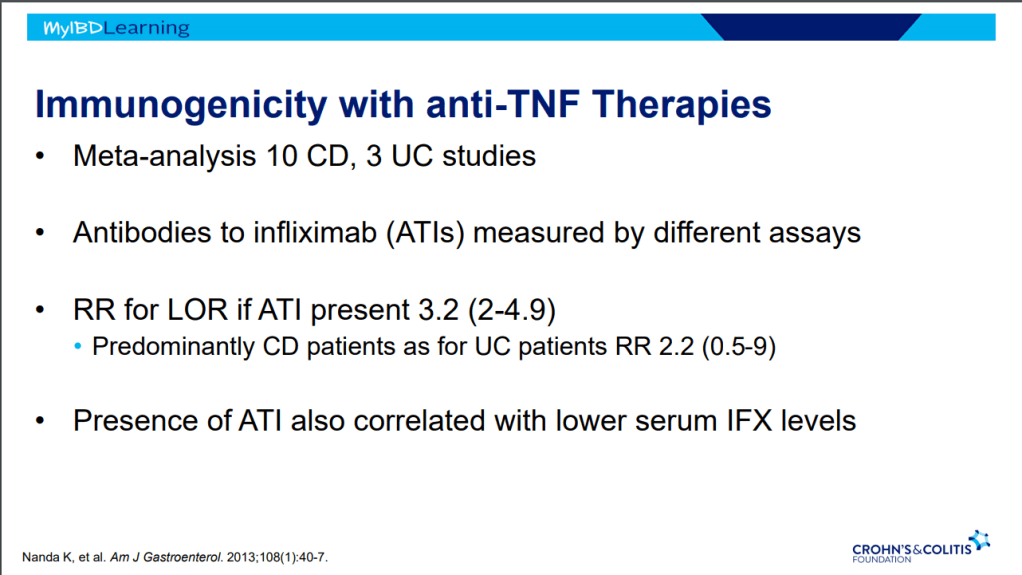
2. S Schreiber et al. Inflamm Bowel Dis 2024; 30: S7. NETWORK META-ANALYSIS TO EVALUATE THE COMPARATIVE EFFICACY OF BIOLOGICS FOR MAINTENANCE TREATMENT OF ADULT PATIENTS WITH CROHN’S DISEASE
Methods: A network meta-analysis (NMA) was conducted to evaluate comparative efficacy of licensed biologics. Phase 3 randomized controlled-trials (RCTs) evaluating biologics approved by the European Medicines Agency or United States Food and Drug Administration as of 31 March 2023 for maintenance treatment of adult patients with moderate-to-severe CD were included, i.e. infliximab (IFX) intravenous (IV) and SC, adalimumab (ADL) SC, vedolizumab (VDZ) IV and SC, ustekinumab (UST) SC, and risankizumab (RZB) SC.
Key findings:
- Among 8 comparator arms, IFX SC 120 mg every 2 weeks (Q2W) showed the highest odds ratio (95% credible interval) vs. PBO for clinical remission during the maintenance phase (3.52 [2.18–5.65]).
My take: This meta-analysis shows a favorable response for IFX SC; however, head-to-head trials are needed to really determine which biologic has the highest efficacy.
3. N Bevers et al. JPGN 2024; 2023; 77: 628-633. Fatigue and Physical Activity Patterns in Children With Inflammatory Bowel Disease
In this cross-sectional study with 104 children (24 with fatigue), biological parameters (CRP, fecal calprotectin) did not discriminate fatigued from non-fatigued patient
4. Y Bouhnik et al. Clin Gastroenterol Hepatol 2023; 21: 3365-3378. Adalimumab in Biologic-naïve Patients With Crohn’s Disease After Resolution of an Intra-abdominal Abscess: A Prospective Study From the GETAID
In this multicenter prospective study with 117 patients, the authors examined the success rate of adalimumab (ADA) in patients with CD with an intra-abdominal abscess resolved without surgery.
Key findings:
- At W24, the survival rate without abscess recurrence or surgery was 74% (n=87)
- Abscess drainage was significantly associated with ADA failure at W24 (odds ratio, 4.18)
My take (borrowed from authors): Provided that the abscess was carefully managed before initiating medical treatment, this study showed the high efficacy of ADA in the short and long term in biologic-naïve patients with CD complicated by an intra-abdominal abscess