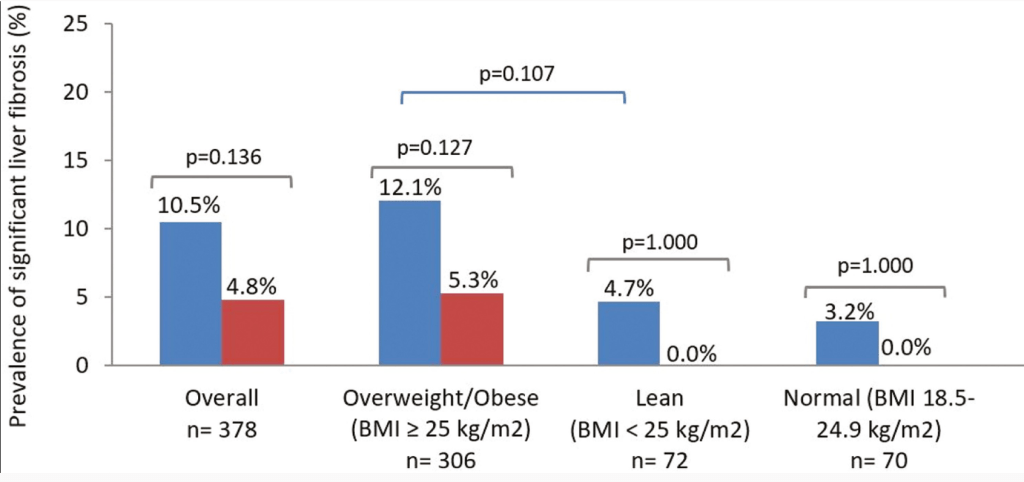
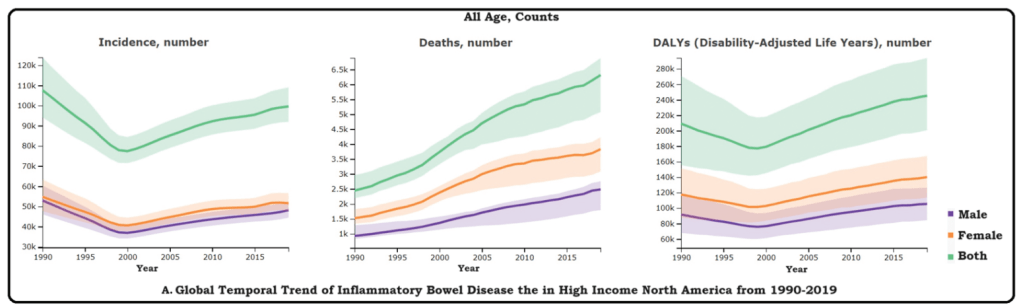
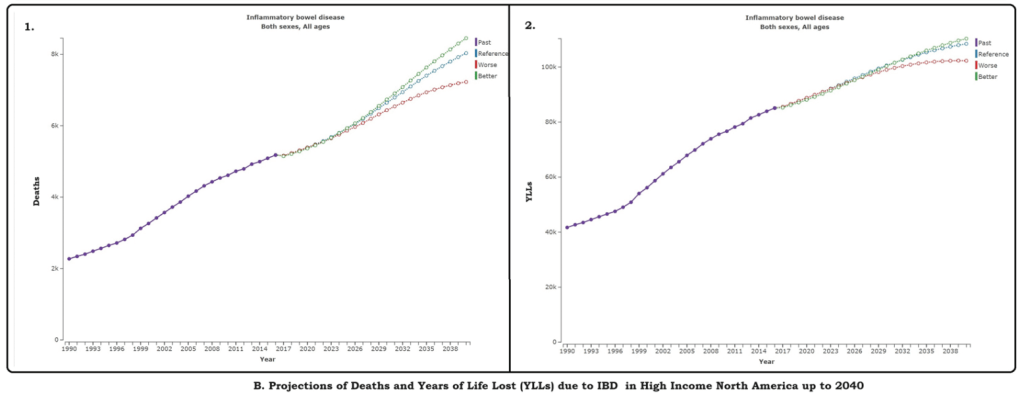
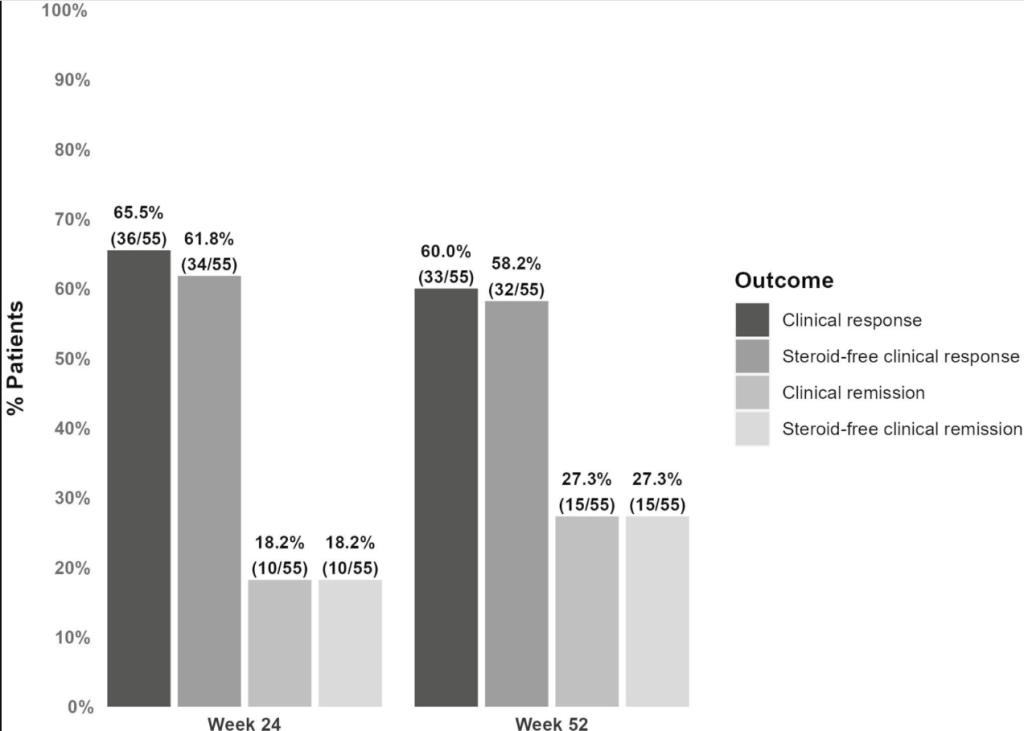
M Agrawal et al. Clin Gastroenterol Hepatol 2024; 22: 2459-2467. Open Access! Breastfeeding Duration Is Not Associated With Offspring Inflammatory Bowel Disease Risk in Three Population-Based Birth Cohorts
The authors utilized prospectively collected data from 3 population-based birth cohorts (Danish National Birth Cohort, Norwegian Mother, Father, and Child Cohort, and All Babies in Southeast Sweden). This collectively included nearly 170,000 offspring.
Key findings:
- During median follow-up of 16.3–22.3 years, between 1996 and 2021, 543 offspring were diagnosed with IBD
- In each country, there was no association between exclusive breastfeeding duration and offspring IBD risk
Discussion:
“In contrast to majority of case-control studies, both cohort studies reported null association between breastfeeding, treated as a binary exposure (any versus no breastfeeding) or by duration, and offspring IBD risk. Similarly, 2 nested case-control studies, leveraging prospectively collected data on early life exposures as part of the population-based Jerusalem Perinatal Study and 2 United Kingdom birth cohorts (the 1946 National Survey of Health & Development and the 1958 National Child Development Study) reported null associations between breastfeeding and IBD risk.22,23 Data from these studies, which are more rigorous in methodology compared with case-control studies, are consistent with findings from our analyses.”
My take: While this study has some limitations inherent in observational data, this study with prospectively-collected data indicates that breastfeeding did not modulate the risk of developing IBD.
Related blog posts:
- Mom, Can We Get a Dog (& a Sibling)? I Don’t Want to Get Crohn’s Disease
- Breastfeeding: Protection from Inflammatory Bowel Disease
- Breastfeeding Associated with Infant Survival
- Guilt of Breastfeeding Failure
- Briefly Noted: Breastfeeding and Microbiome Diversity
- With Regard to Avoiding Excessive Weight Gain, Breastfeeding is Best
- “The 10 Steps to Successful Breastfeeding”
- Breastfeeding and IQ -the Latest Data