Peyrin‐Biroulet, L., Arkkila, P., Armuzzi, A. et al. BMC Gastroenterol 24, 121 (2024). https://doi.org/10.1186/s12876-024-03163-5. Open Access! Comparative efficacy and safety of subcutaneous infliximab and vedolizumab in patients with Crohn’s disease and ulcerative colitis included in randomised controlled trials.
Methods: Studies included in the current analysis were parallel-group, randomised controlled trials (RCTs) that evaluated treatment with IFX SC, following induction therapy with IFX IV, or treatment with VDZ (either with VDZ IV or with VDZ SC [following IV induction therapy]). The authors identified three eligible CD trials and four eligible UC trials that assigned over 1200 participants per disease cohort to either IFX SC or VDZ.
Key findings:
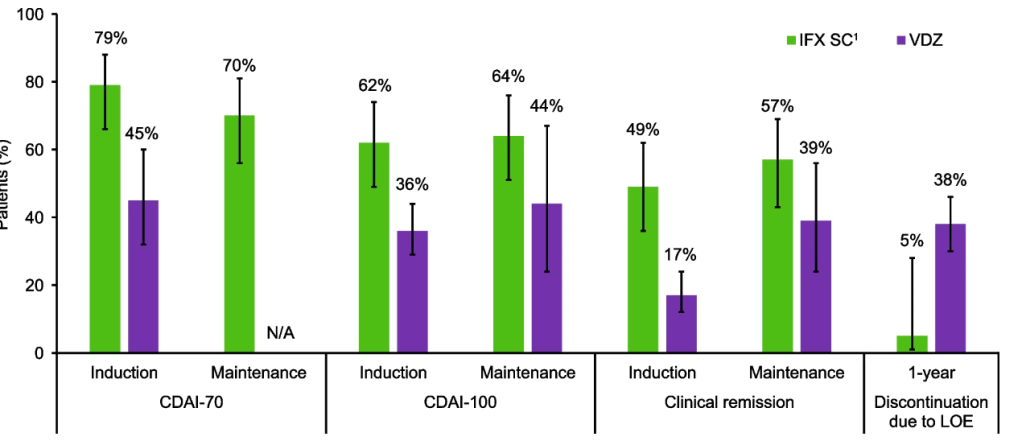
Crohn’s disease: Intravenous induction therapy with IFX demonstrated better efficacy compared with VDZ; during the maintenance phase, IFX SC showed numerically better efficacy than VDZ. A lower proportion of IFX SC-treated patients discontinued therapy due to lack of efficacy over 1 year.
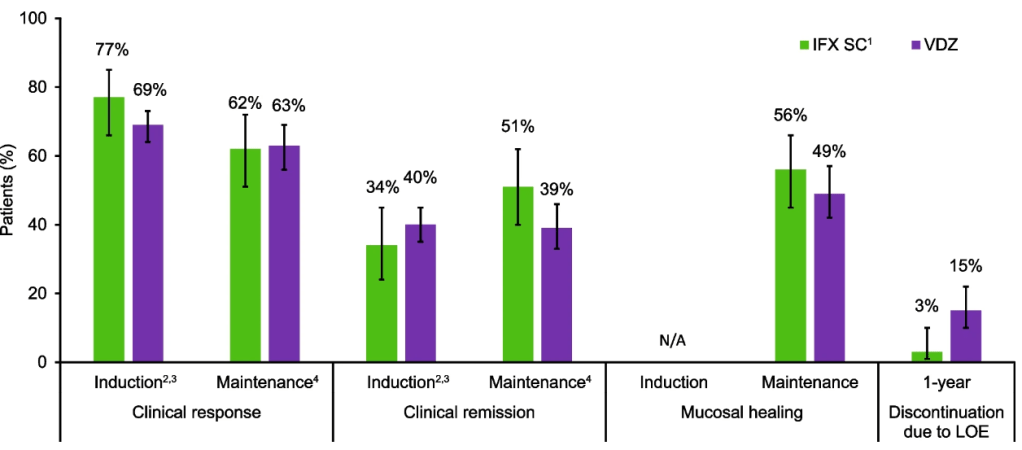
Ulcerative colitis: Efficacy profiles were similar with IFX SC and VDZ during the induction and maintenance phases, and a lower proportion of IFX SC-treated patients discontinued therapy due to lack of efficacy over 1 year.
Safety: In both cohorts, safety profiles for IFX SC and VDZ were generally comparable during 1 year.
Discussion Points:
- The authors discuss some limitations of their study. “The GEMINI I, GEMINI II, and VISIBLE 1 trials were rated as being at high risk of bias for the category ‘other’ bias, because only patients who achieved a clinical response during induction went on to participate in the maintenance phase, which could potentially lead to a higher estimate of efficacy during the maintenance phase than if patients who did not achieve a clinical response were also included.”
- The vedolizumab studies notably included a high proportion of patients who failed to respond to anti-TNFs. “All VDZ studies permitted enrolment of patients with prior TNFi failure, accounting for 47.5% of VDZ-treated patients overall.” Thus, in a true head-to-head study with patients unexposed to biologics, VDZ may achieve better results.
My take: This study indicates that SC infliximab (like IV infliximab) appears to be more effective than vedolizumab for patients with Crohn’s disease and similarly effective for ulcerative colitis, keeping in mind the aforementioned discussion points. While not evident in this study, vedolizumab has a superior safety profile.
Related blog posts:
- Vedolizumab and Infliximab: Expected Dosing When Switching From IV to SC Routes
- REMSWITCH: Infliximab IV to SC Study
- Subcutaneous Vedolizumab Now FDA-Approved for Adults with Ulcerative Colitis
- IBD Updates: SC Vedolizumab, PRODUCE study: Specific Carbohydrate Diet, Racial Epidemiology of IBD, and Microbiome in UC
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.