I recently attended a regional CCFA conference. David Rubin gave several terrific lectures. Here are some of my notes and some slides from this lecture. My notes may contain transcription errors as well as important omissions. Can get access to full set of slides here: Biologics and Their Biosimilars
“Biologics and Their Biosimilars“
What is a Biologic Therapy?
Dr. Rubin makes a point of explaining the term to patients. It is a protein made in a living cell that targets another protein. Term “biologic” can sound scary to patients. Usually given IV because they cannot be absorbed through the small bowel.
IBD Treatment Revolutions
- Steroids -overnight changed mortality in IBD
- Anti-TNF Therapy in IBD -taught many lessons. Treat earlier –>better outcomes.
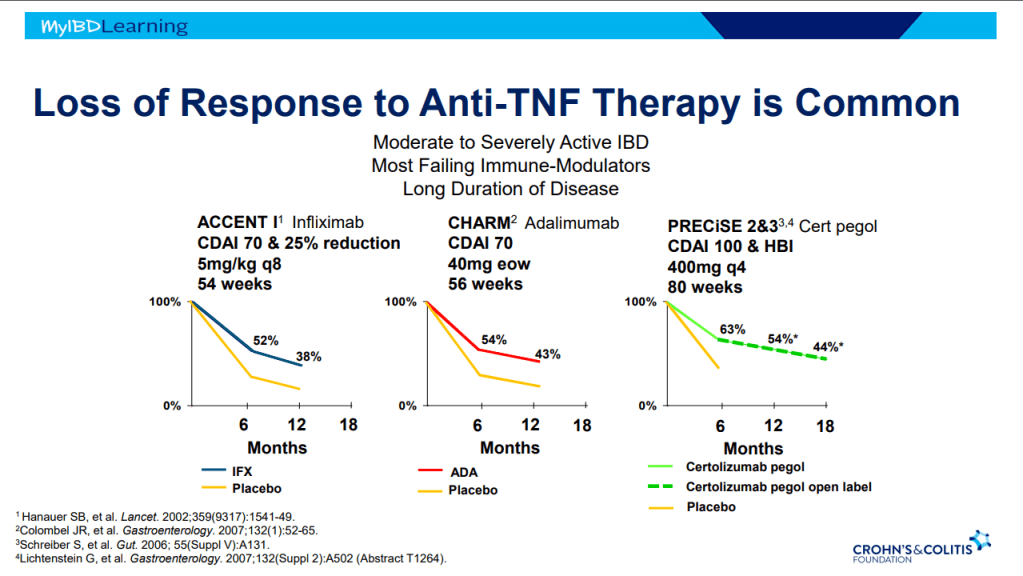
Anti-TNF Therapy
- Frequent loss of response.
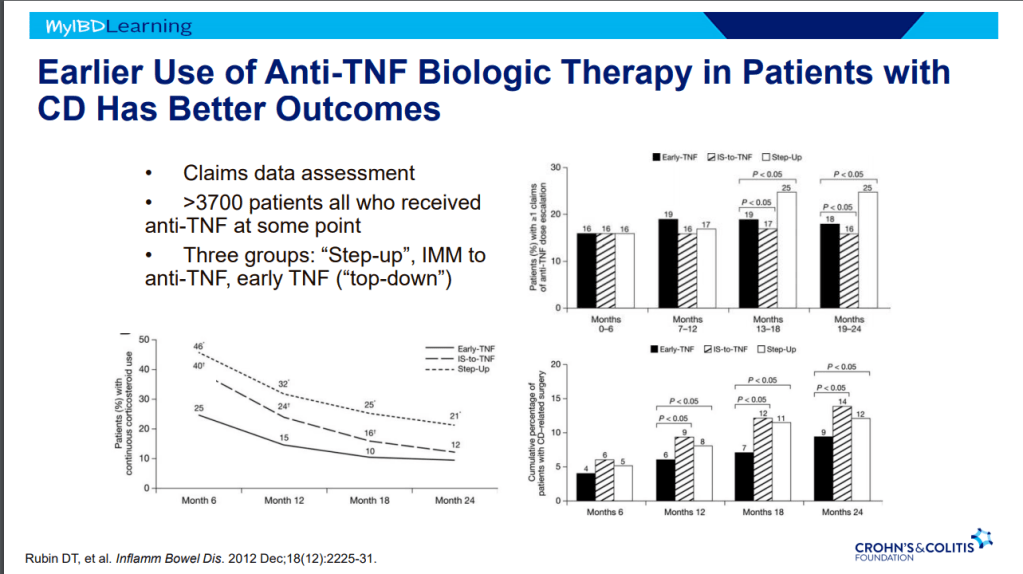
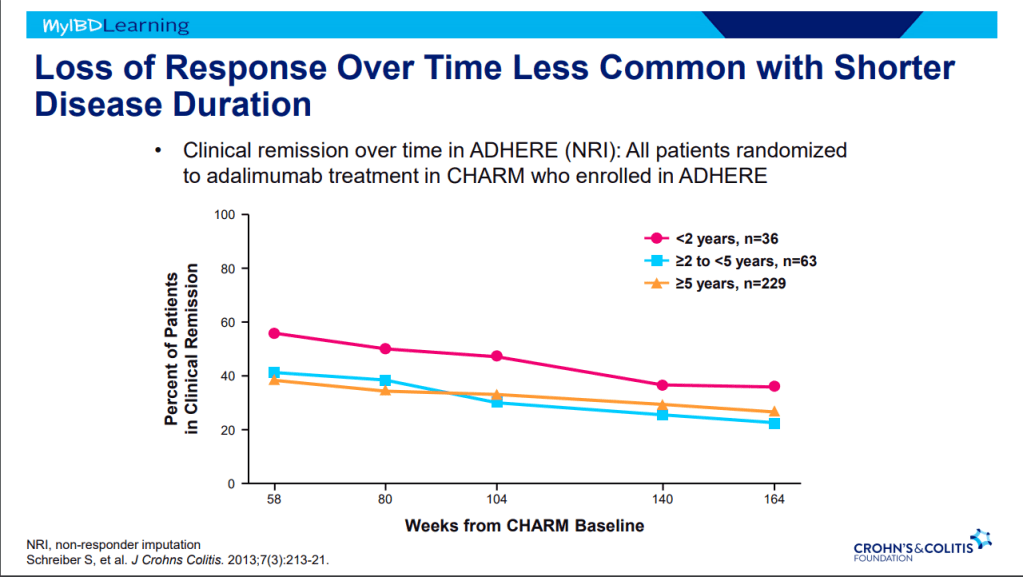
- Earlier treatment with biologics result in better outcomes.
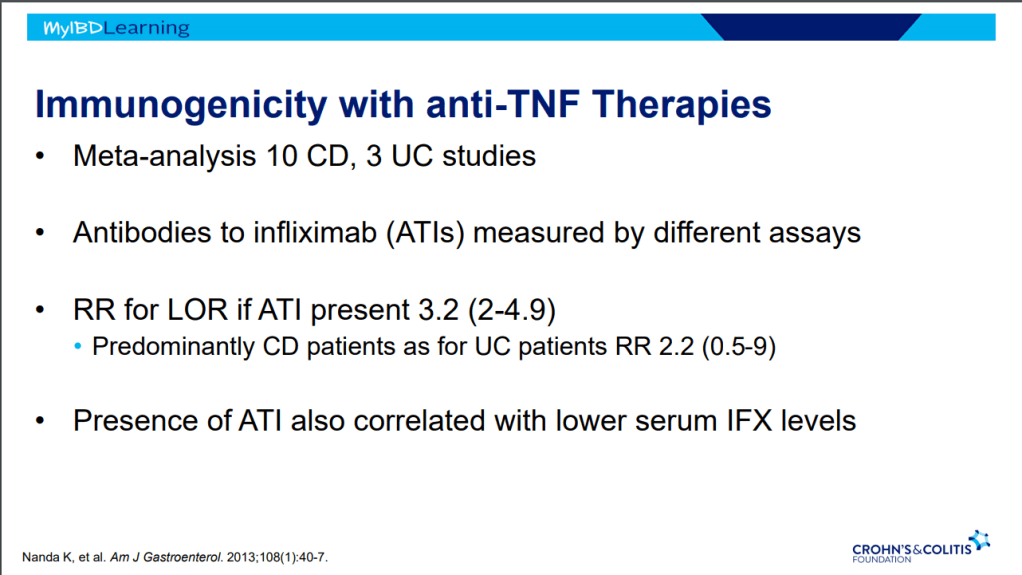
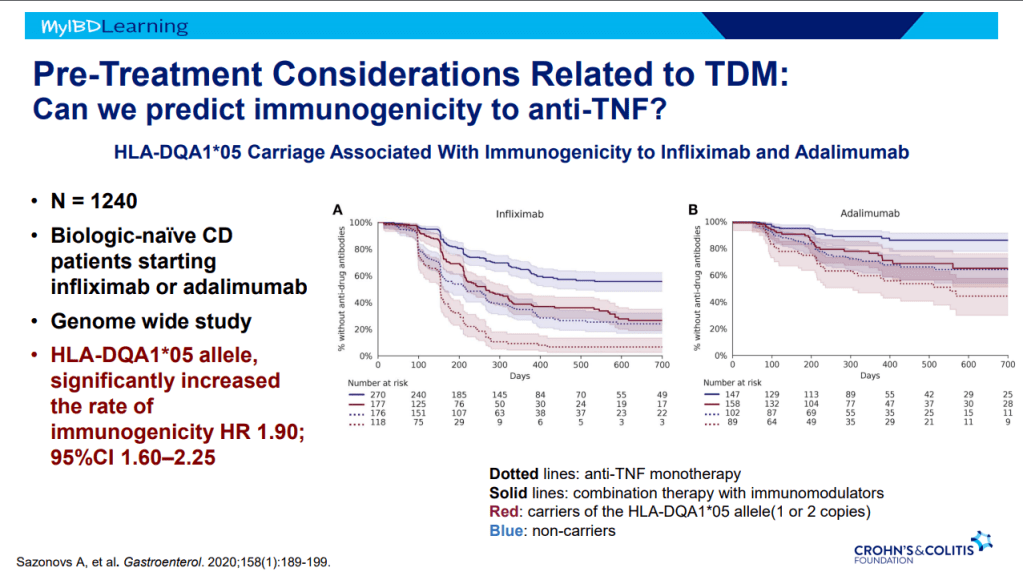
- Immunogenicity is mainly an issue with anti-TNF agents and not much of an issue with other biologics. Episodic therapy is a big risk factor for anti-drug antibodies.
- If staying with in-class medication, after anti-drug antibodies, need to take additional measures to prevent anti-drug antibodies (eg. Immunomodulators).
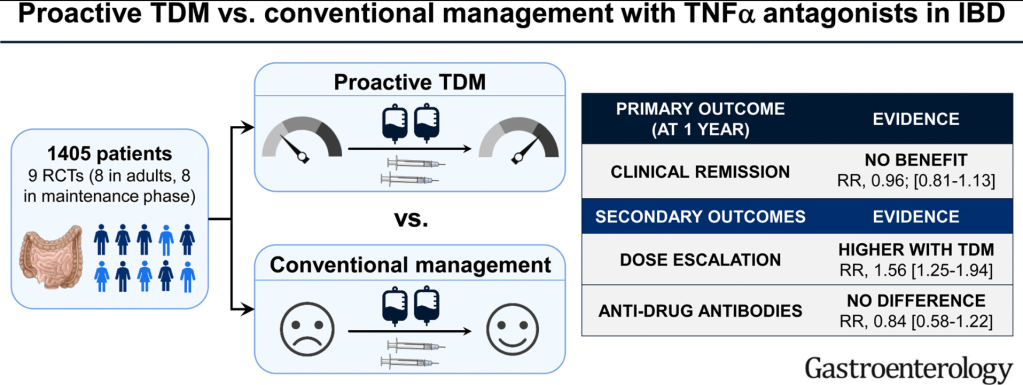
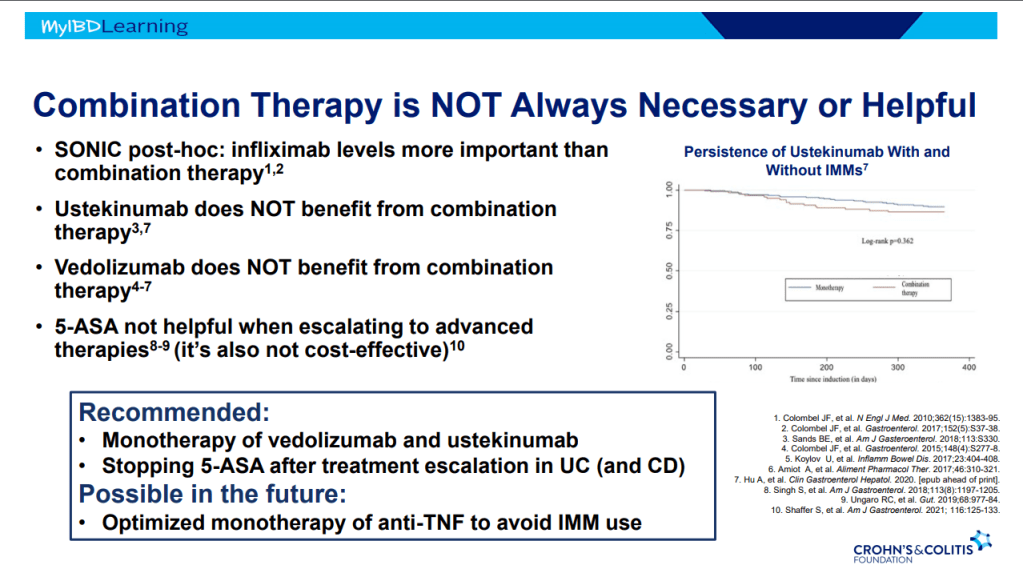
- Combination therapy is more effective (SONIC, UC SUCCESS trials). This is due to using multiple mechanisms of disease control, reduction in anti-drug antibodies, and elevated serum drug levels.
- Good therapeutic levels appears to deliver similar results as combination therapy
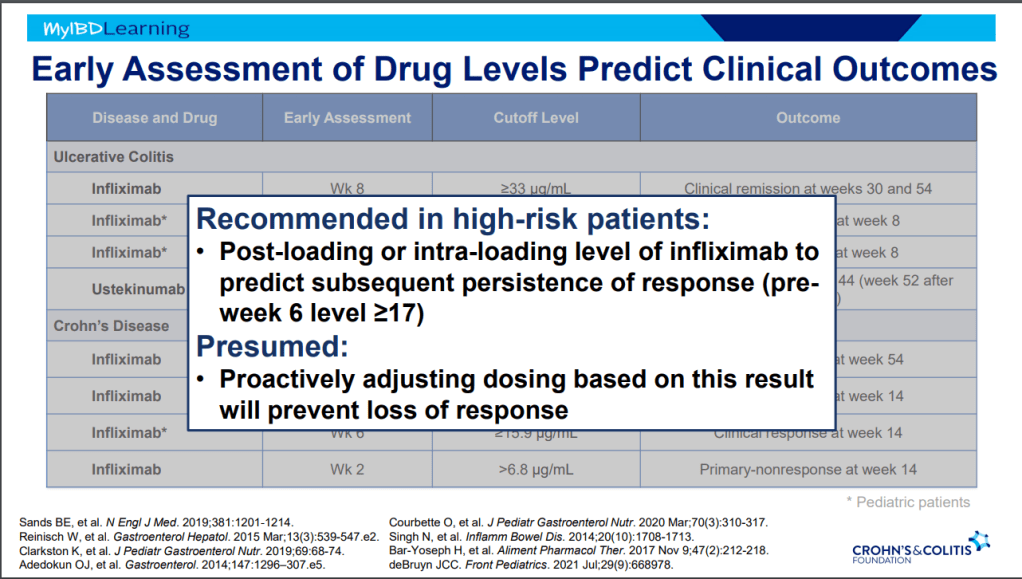
- Pre-week 6 level of 17 or greater, associated with good response in maintenance. If level is low, presumption is that higher dosing will be beneficial.
- Higher levels of infliximab trough levels needed for perianal fistula healing (improved with ciprofloxacin). Higher levels could be causally-related to healing or could be a marker that there is less inflammation and a patient is responding.
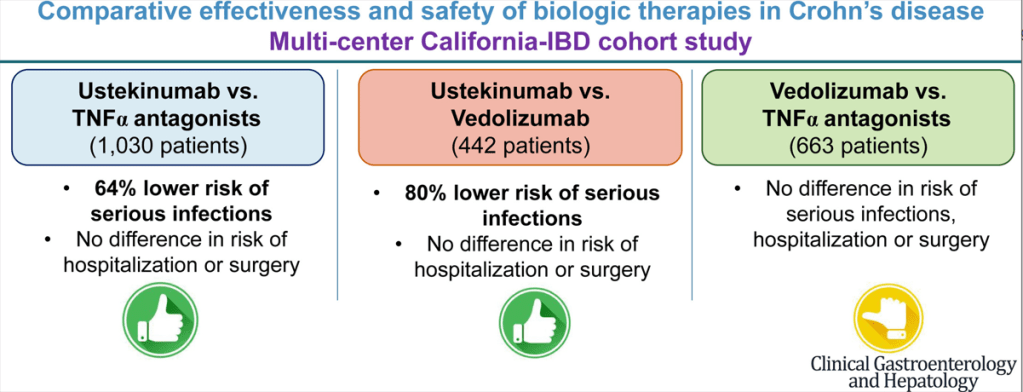
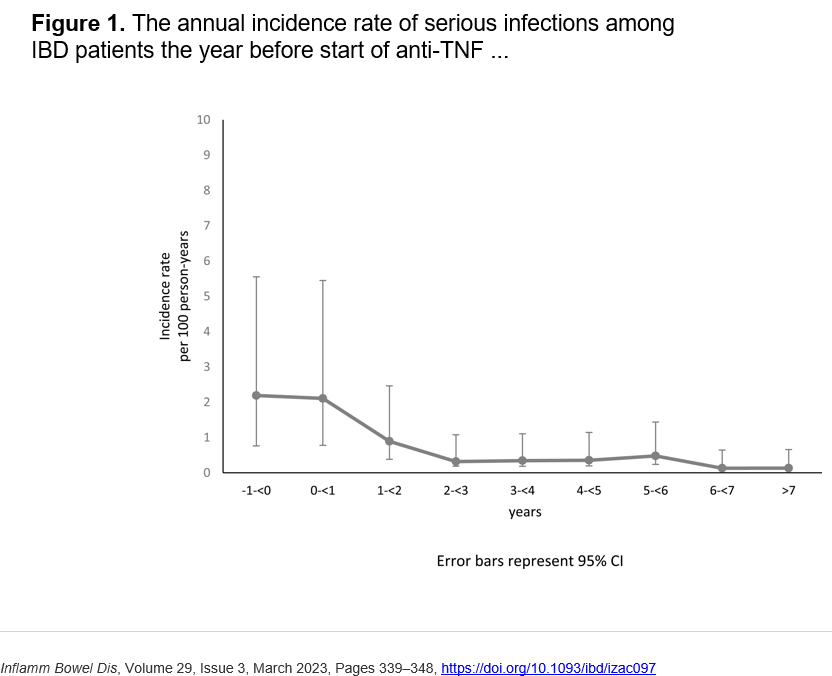
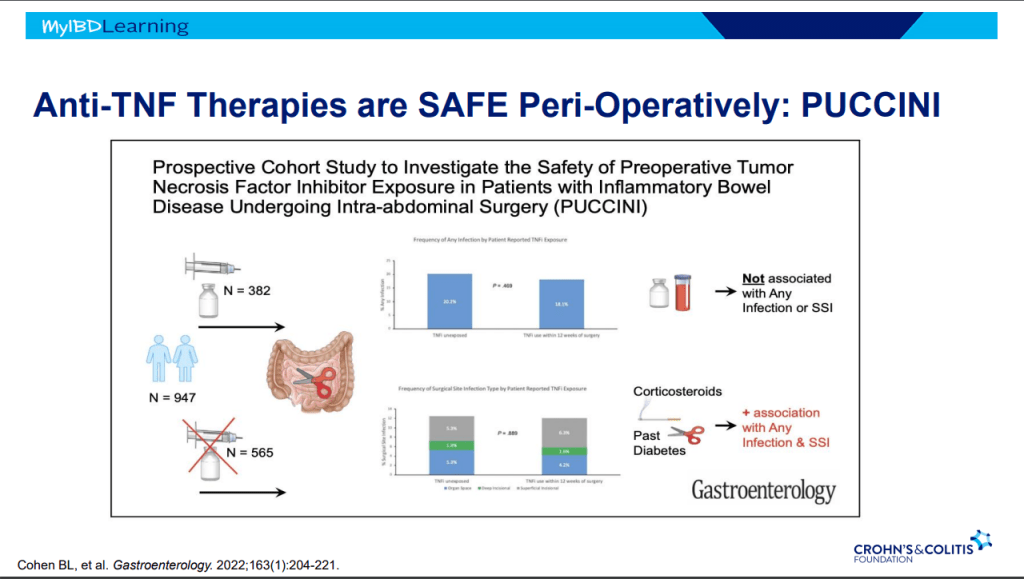
- Anti-TNFs do not appear to increase risk of infections (see PUCCINI study)
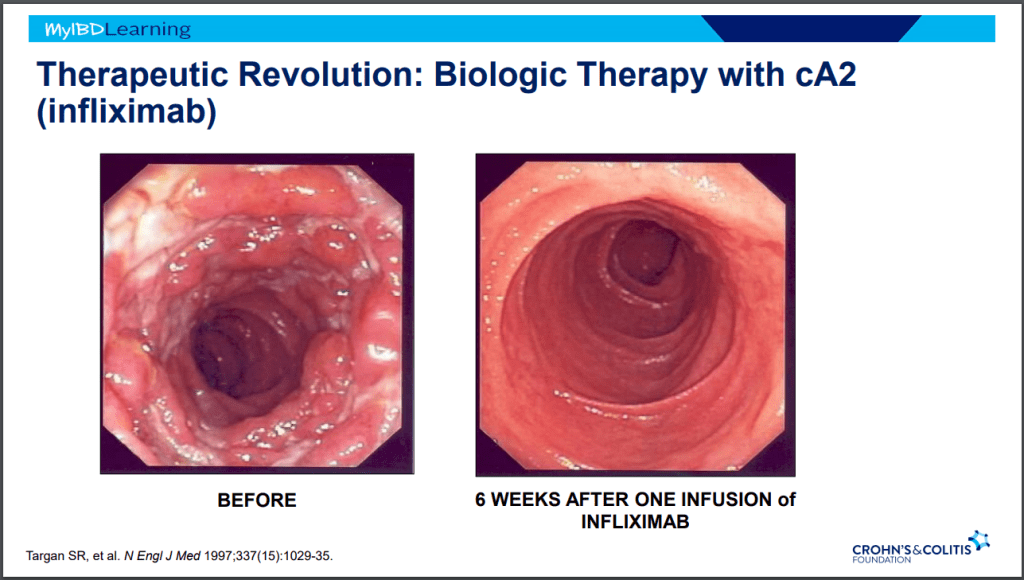


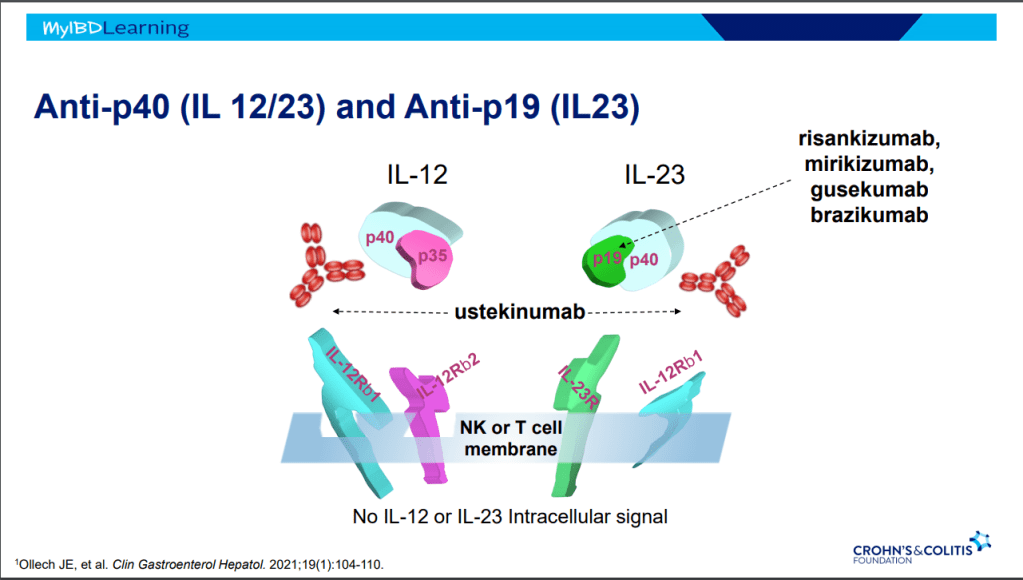
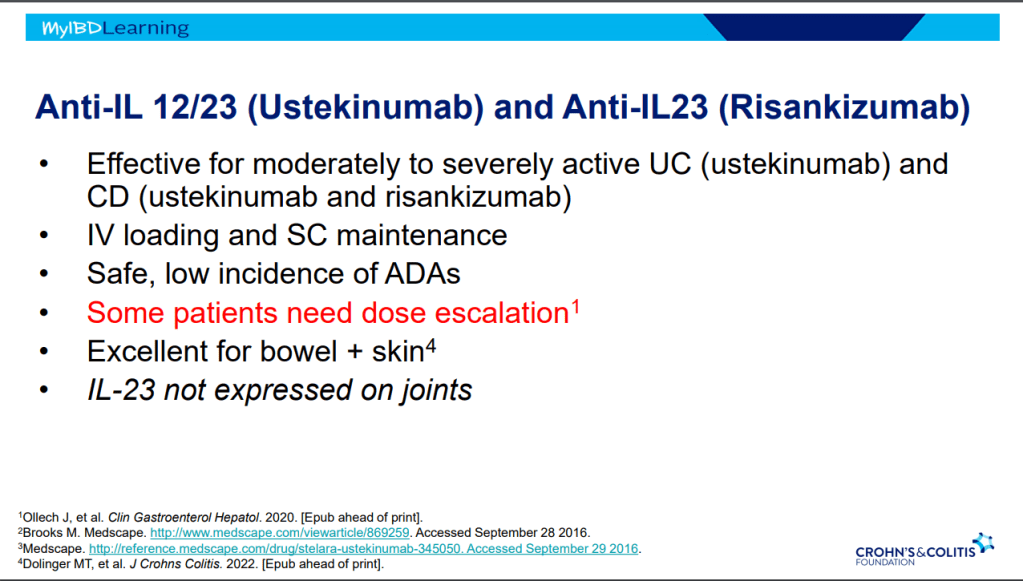
Anti-23 and Anti-IL-12/IL-23
- Tissue selective targeted therapy –>excellent safety profile
- IV loading and SC maintenance
- Excellent for bowel and skin
- IL-23 is not expressed in joints
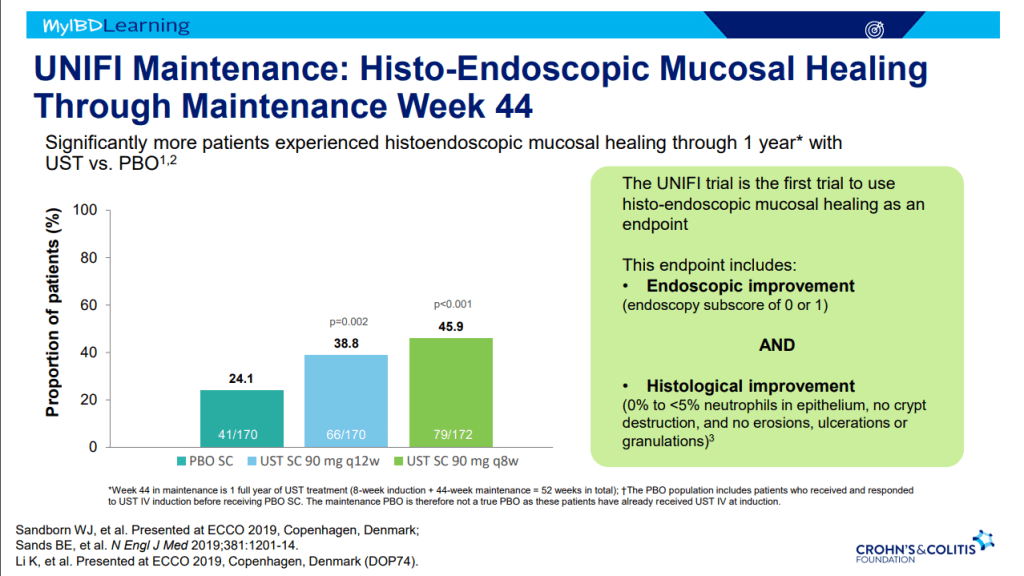
- Ustekinumab is effective for perianal disease and ulcerative colitis
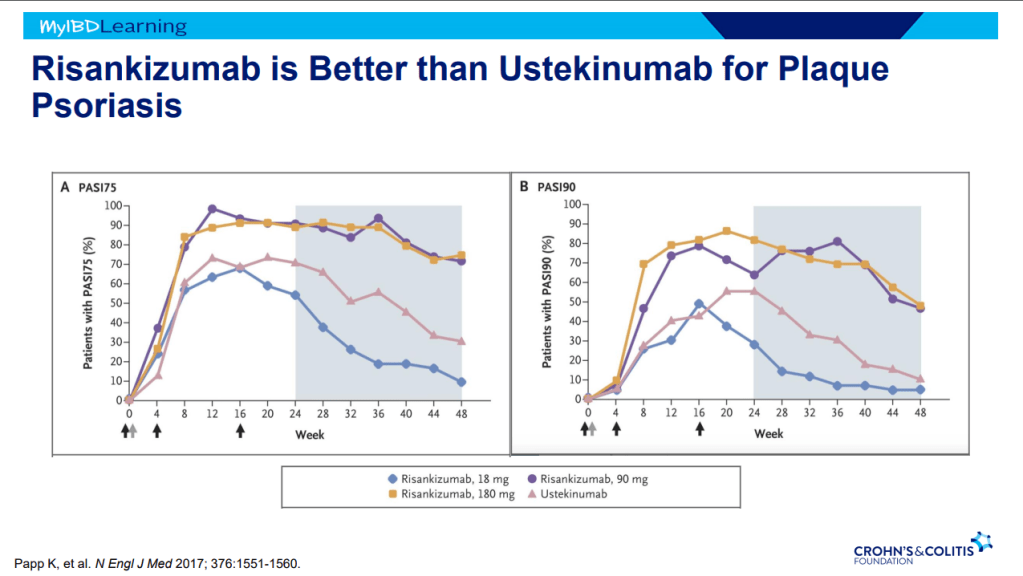
- Risankizumab is superior to ustekinumab in plaque psoriasis. If loss of response to ustekinumab, can still respond to Risankizumab
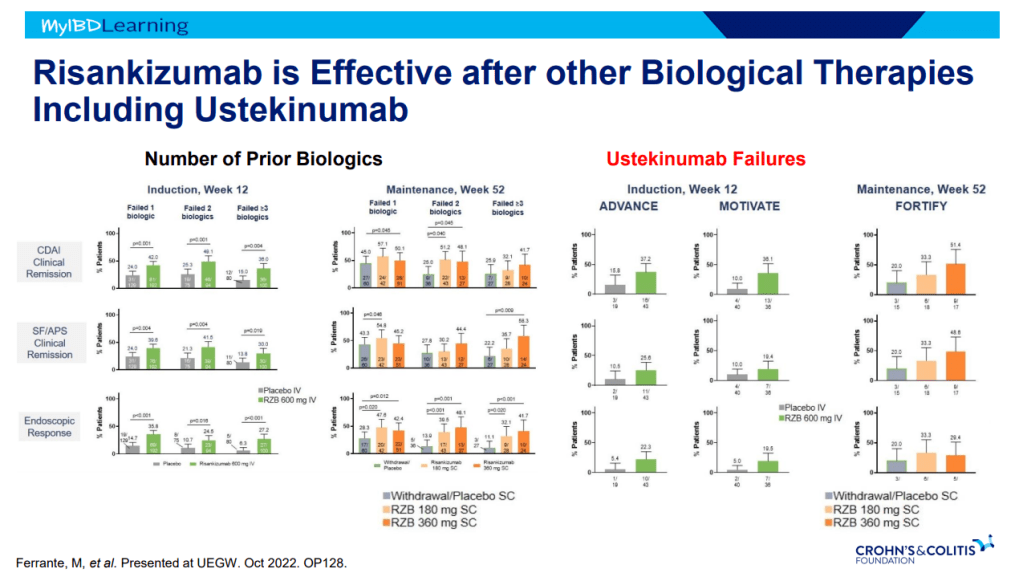
Anti-Integrins:
- Natalizumab (not used frequently in IBD)
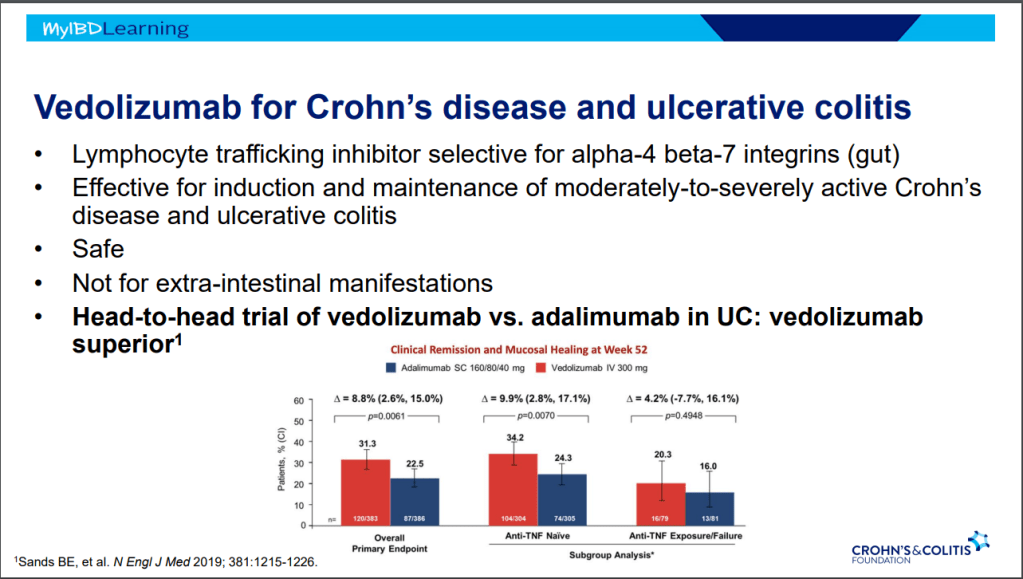
- Vedolizumab. Affects mucosa (can explain frequent nasopharyngitis)
- Vedolizumab -terrific safety profile. No PML, no malignancy risk
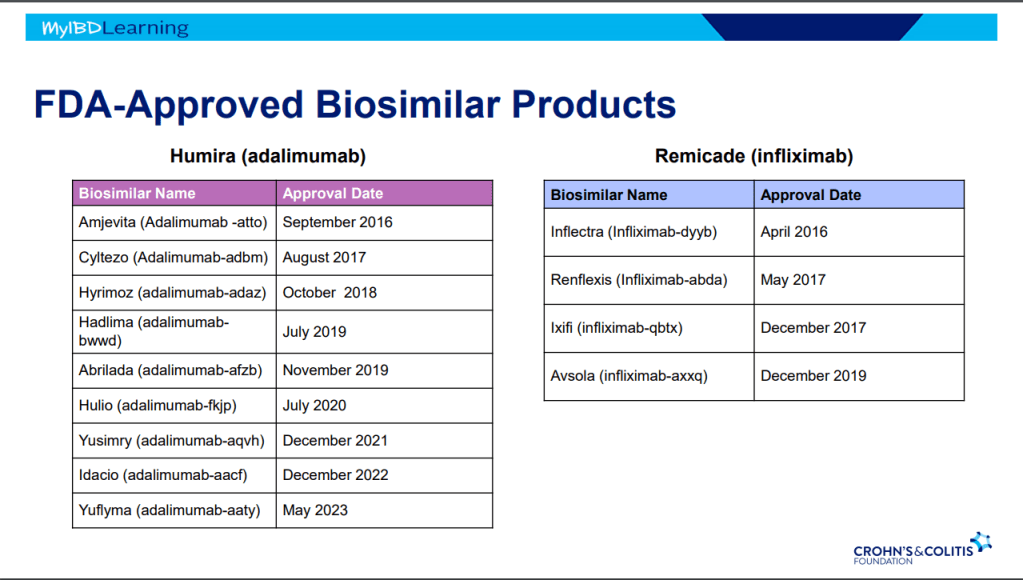
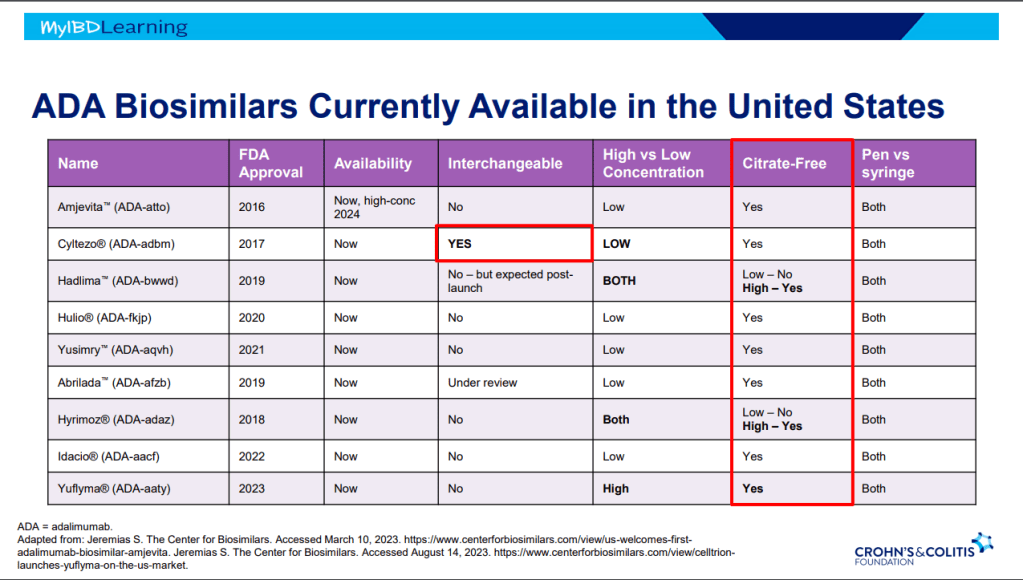
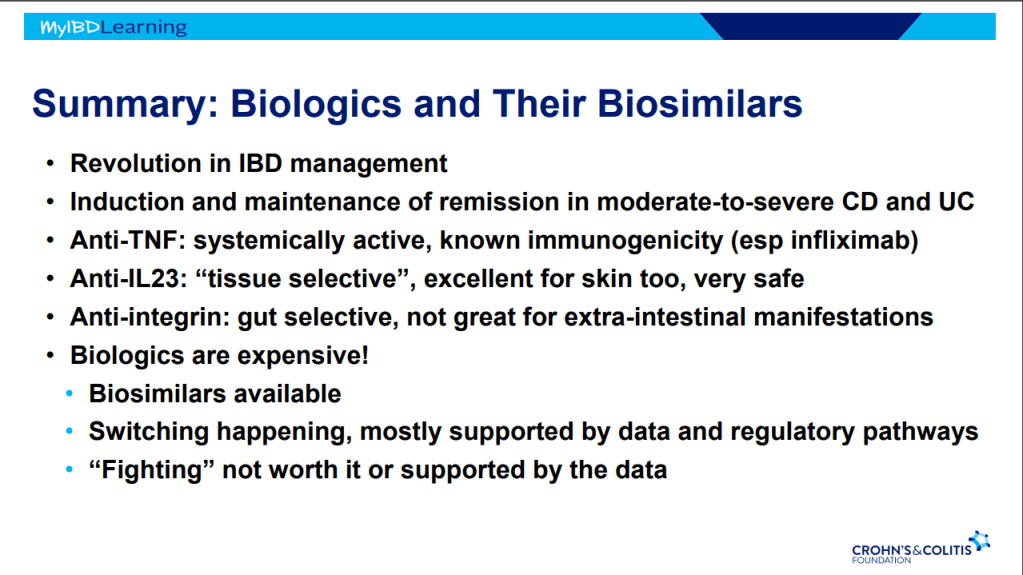
Biosimilars:
- If biosimilar found effective for one approved condition, extrapolation given to all indications
- IBD switching studies have NOT shown increased loss of response. Consider reassess prior to switch to help determine if patient truly in remission prior to switch. Switching often blamed for loss of response when many times the disease was not under good control prior to switch
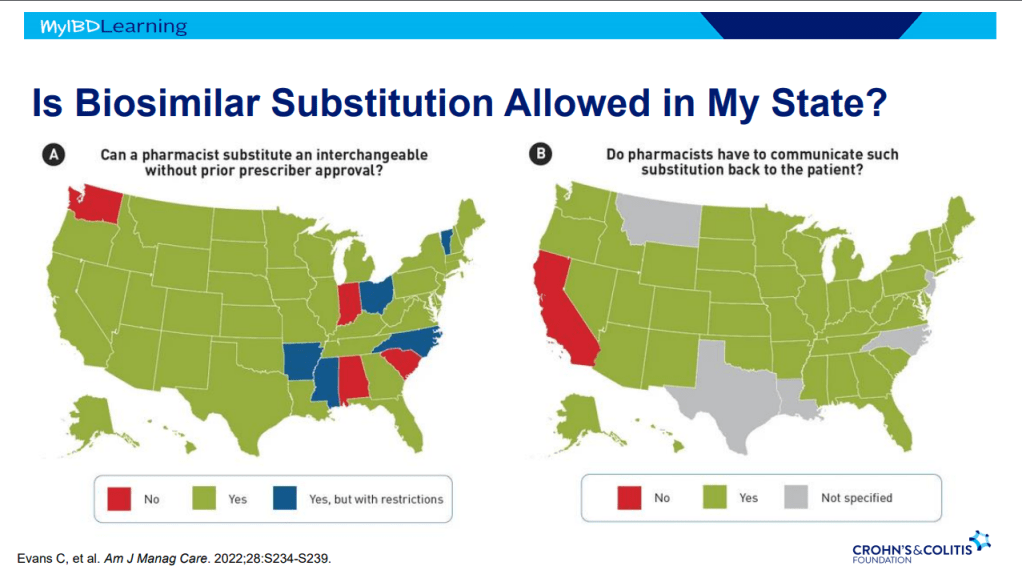
- Interchangeable indicates that the drug can be switched by pharmacists
- Biosimilars are saving insurers money but no proof that this is saving patients money
- Anti-drug antibodies will cross-react to biosimilars
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.