A Yerushalmy-Feler et al. Inflammatory Bowel Diseases, 2025, 31, 3320–3326. Real-World Experience with Upadacitinib for Pediatric Acute Severe Ulcerative Colitis: An International Multicenter Retrospective Study from the Pediatric IBD Porto Group of ESPGHAN
In this study of 22 pediatric patients with ASUC refractory to infliximab, key findings:
- By week 26, 14 (64%) were in corticosteroid-free clinical remission and 16 (73%) patients remained colectomy-free
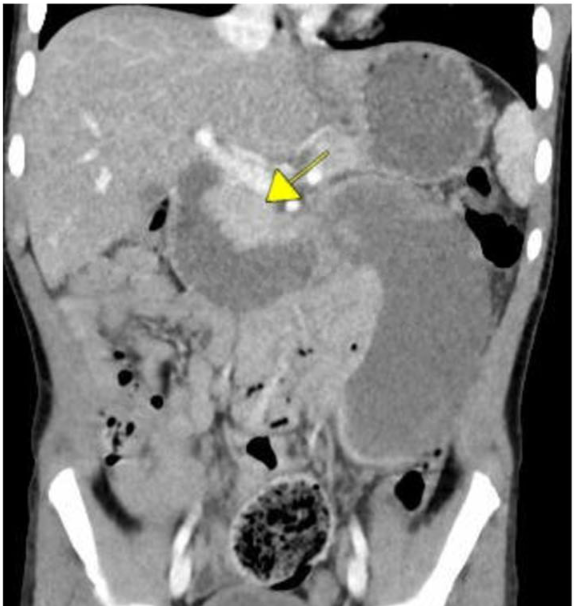
- Two serious AEs of an appendiceal neuroendocrine tumor and cytomegalovirus colitis
My take: It is good to see more pediatric data. The availability of upadacitinib will likely lead to lower colectomy rates.
Related blog post: IBD Briefs: Upadacitinib in Children, Predicting Crohn’s Disease, and Autoimmune Diseases Associated with IBD
L Bertani et al. Inflammatory Bowel Diseases, 2025, 31, 3363–3369. When to Switch to Subcutaneous Infliximab? The RE-WATCH Multicenter Study
Methods: The RE-WATCH study was an observational, multicenter, retrospective study performed in four IBD referral centers. Inclusion criteria meant that only patients receiving on label SC-IFX at a dosage of 120 mg every other week were included in the study. The initiation of IFX therapy as the baseline timepoint.
Key findings:
- There were no statistical differences between the two groups, early vs. late switch, after one year in terms of the respective endoscopic response (71.4% vs 70.8%, P = .95), steroid-free clinical remission (62.5% vs 68.7%, P = .51), or IFX retention rate (75.0% vs 66.7%, P = .35).
- There was higher endoscopic remission rates in early switch patients as compared to late switch patients; however, this trend was not significant (69.6% vs 52.1%, P = .07).
- A return to IV-IFX was required in 1 of 43 early switch patients and in 3 of 44 late switch patients (2.3% vs 6.8%, P = .31)
- While the early switch group appears to fare a little better, there is likely a selection bias. For example, the early group had a much lower rate of severe endoscopic score at baseline (20% vs. 54%) and lower rate of Crohn’s fistulizing disease (8% vs 33%).
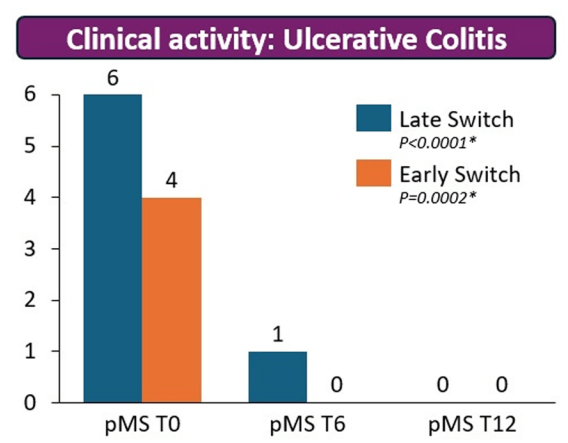
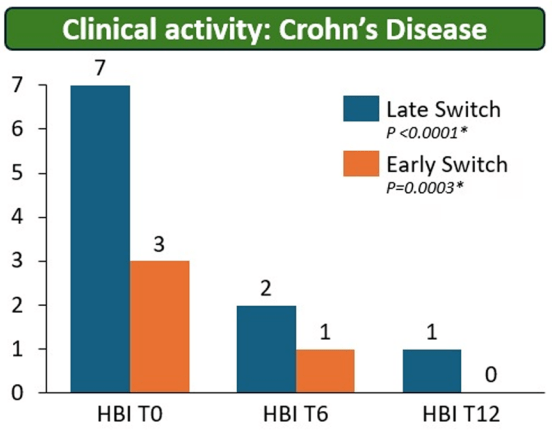
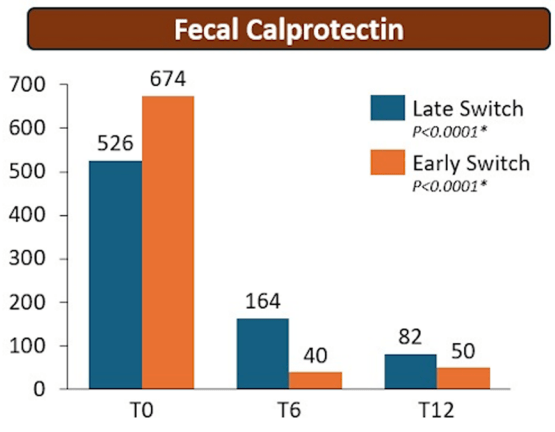
My take: These results indicate that outcomes are similar between patients switching from to IFX SC at both early (after induction) and late (after 6 months).
It is worth noting that prior studies have shown that home-based therapies (eg. home infusion), compared to office-based therapies, have been “associated with suboptimal outcomes including higher rates of nonadherence and discontinuation of infliximab.” This is a concern for SC biologics as well.
Related blog posts:
- LIBERTY Trials for Subcutaneous Infliximab
- Infliximab Thresholds with Subcutaneous vs Intravenous Administration for Crohn’s Disease
- SC Infliximab versus Vedolizumab for Crohn’s Disease and for Ulcerative Colitis
- Vedolizumab and Infliximab: Expected Dosing When Switching From IV to SC Routes
- REMSWITCH: Infliximab IV to SC Study