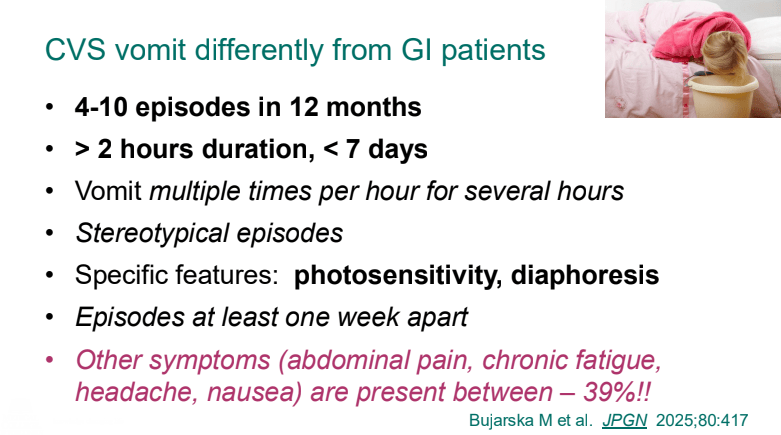
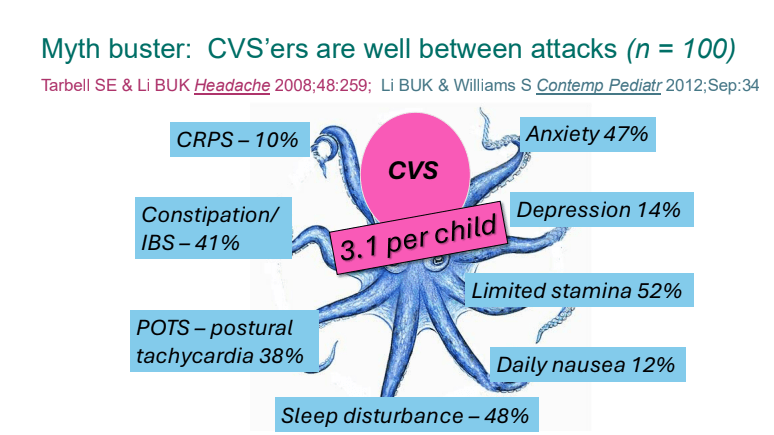
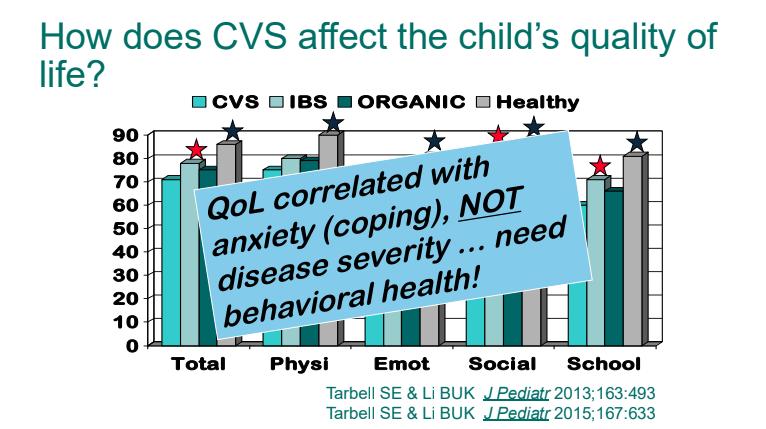
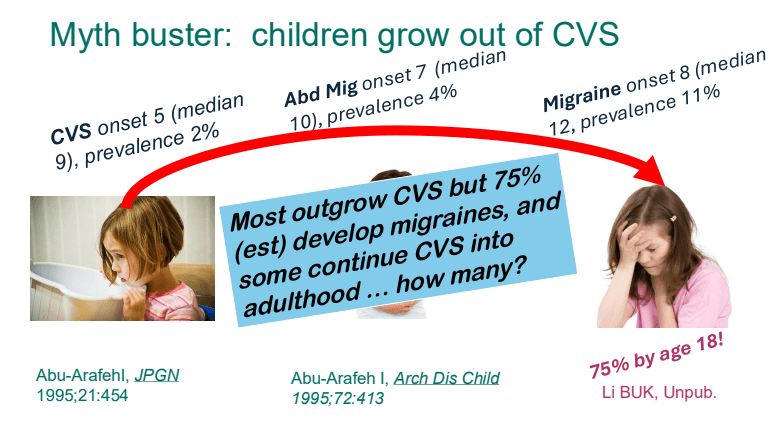
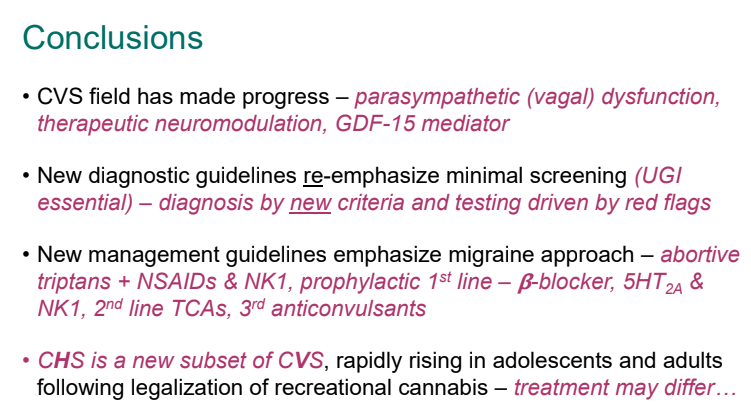
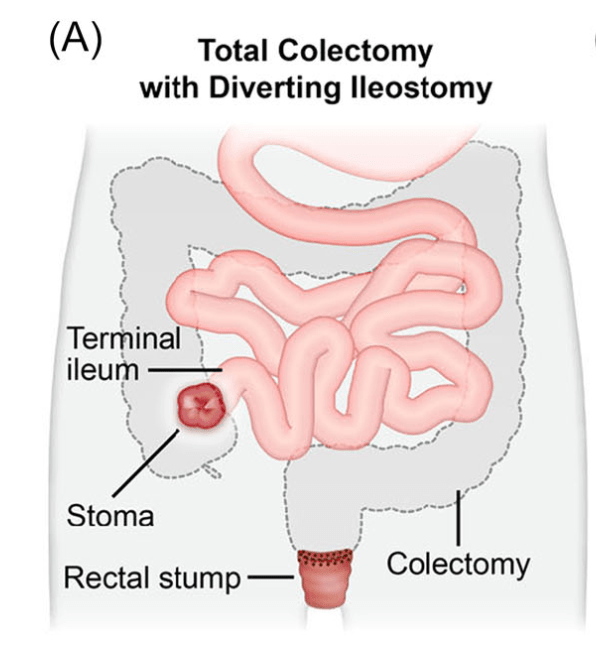
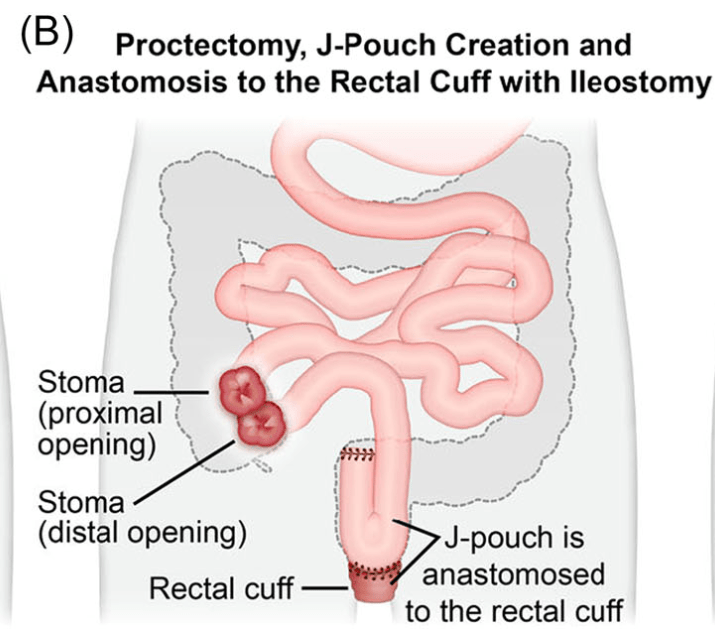
C Strong, ACG 10/28/25: Long-Term PPI Treatment Linked to Higher Risk for Early-Onset CRC Before Age 50
An excerpt:
“Long-term treatment with proton pump inhibitors (PPIs) is independently associated with an increased risk for early-onset colorectal cancer (EOCRC) among individuals aged younger than 50 years, according to study results presented at the American College of Gastroenterology (ACG) 2025 conference…”
“Researchers reviewed data from the National Inpatient Sample from 2016 to 2020. Individuals were aged 18 to 49 years and had a main diagnosis of colorectal cancer (CRC)…Investigators identified PPI exposure through diagnostic codes indicating long-term use (Z79.891) or adverse effects (T45.4X5A/D)….
“Of the 7140 hospitalizations for patients with EOCRC aged younger than 50 years, 1056 (14.8%) reported long-term PPI treatment. After multivariable adjustment, PPI users had a 41% increased risk for EOCRC vs individuals without PPI use (adjusted odds ratio [aOR], 1.41…”
My take: More studies will be needed to determine if this link between PPI use and early onset CRC is truly significant. Many prior associations between PPI and other health conditions on observational studies have not held up with well-controlled studies. There was no increased risk of cancer in a previous randomized control trial (see below).
Related blog posts:
- Meta-Analysis: PPIs Did Not Increase Risk of Cardiovascular Events
- PPIs: Good News on Safety In the only large randomized controlled study (>17,000 patients over 3 years) of PPIs, there was no difference in pneumonia, Clostridium difficile infection, fracture, gastric atrophy, chronic kidney disease, dementia, cardiovascular disease, cancer, hospitalizations, and all-cause mortality in the PPI compared with the placebo arms. ”Enteric infections, which were slightly more common in patients randomized to PPIs, but even there the NNH was more than 900 per year.”
- Thanks: Updated Long-Term PPI Use Smartphrase
- Which Proton Pump Inhibitor is the Most Potent?
- PPIs: Good News on Safety (Part 2) | gutsandgrowth
- Why Observational Studies Are Misleading & PPI Association with Kidney Stones
- Deconstructing PPI-Associated Risks with Nearly 8 Billion Data Points and More on COVID-19 GI Symptoms (Video)
- Austin Bradford Hill, PPIs and IBD
- More Good News for PPIs: NO Increased Risk of Dementia
- PPIs and Associated Heart Risk
- PPI Side Effects: “Dissecting the Evidence” | gutsandgrowth