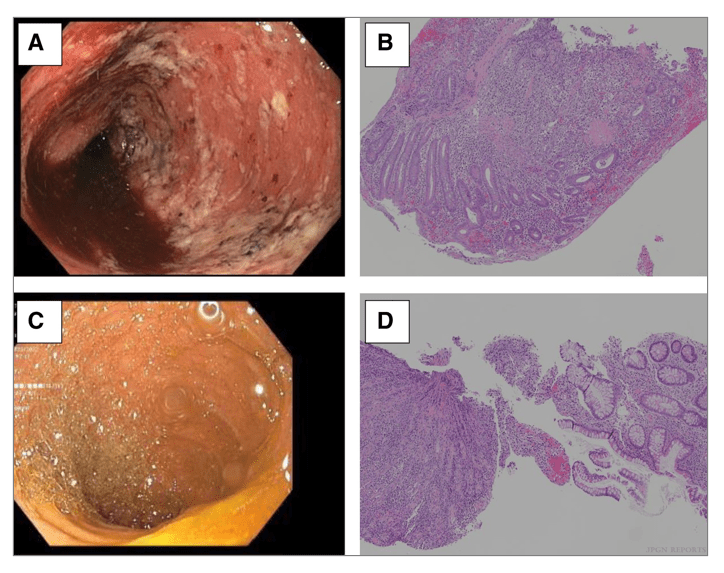
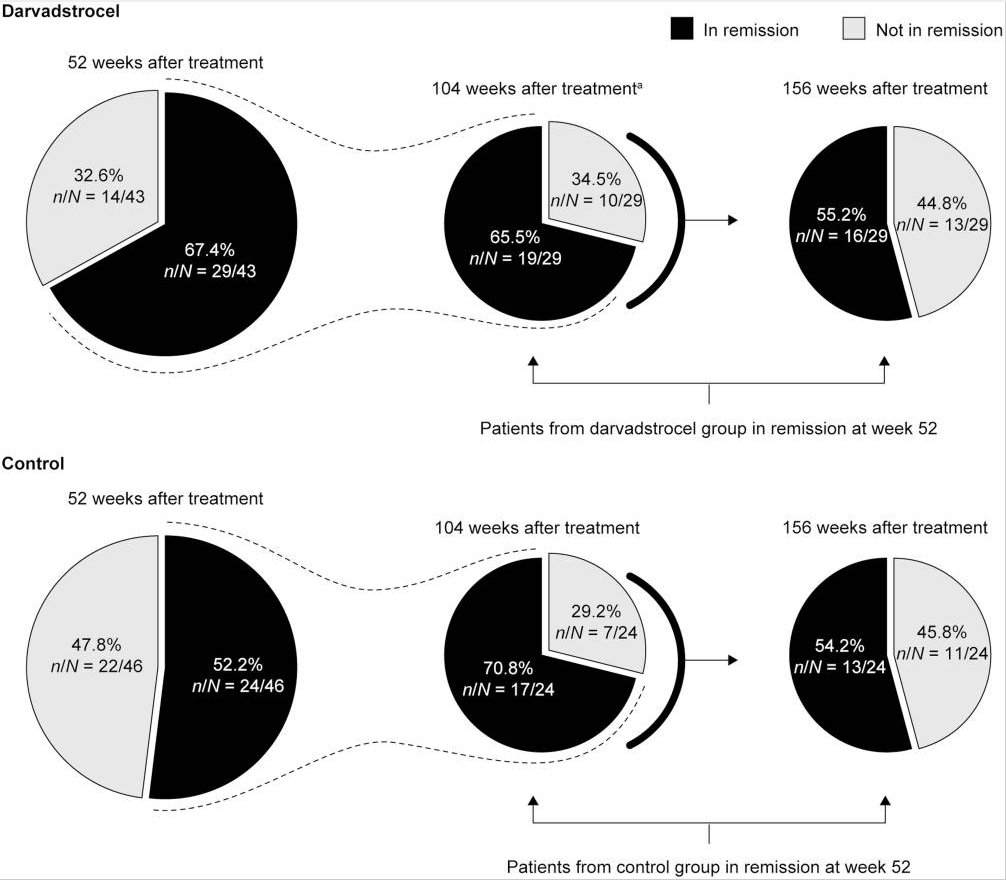
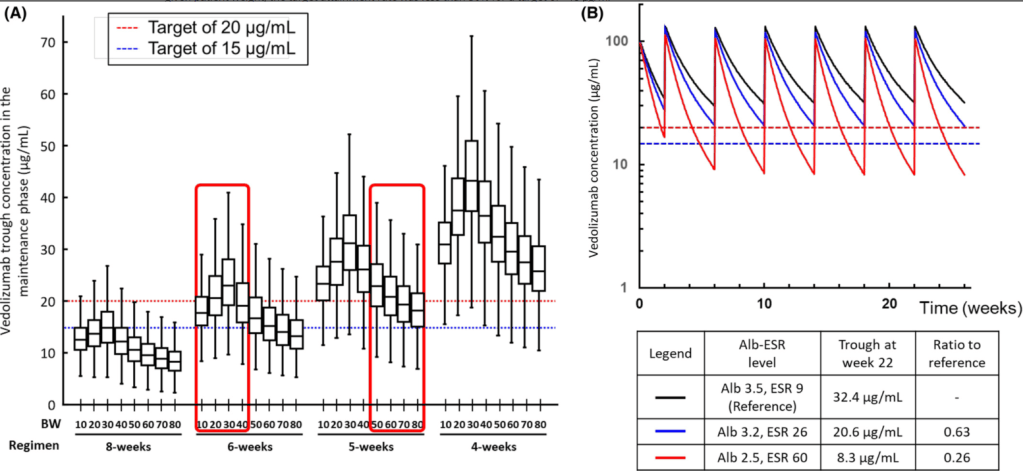
M Agrawal et al. Gastroenterol 2022; 163: 1547-1554. Open Access! The Rising Burden of Inflammatory Bowel Disease in Denmark Over Two Decades: A Nationwide Cohort Study
Key findings:
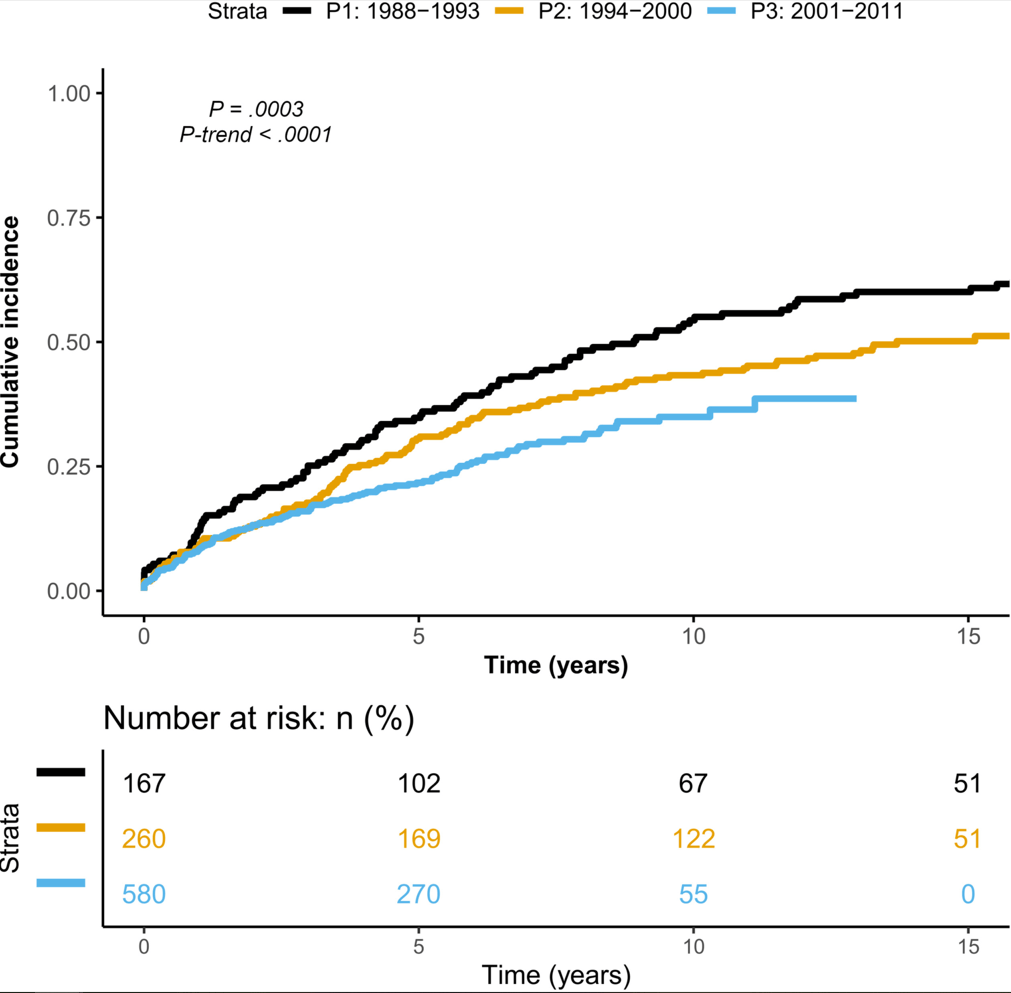
- Between 1995 and 2016, the incidence rate (95% confidence interval) per 100,000 person-years rose from 9.1 (8.3–10.0) to 17.8 (16.8–19.0) for CD, and from 21.0 (19.8–22.3) to 28.4 (27.0–29.8) for UC.
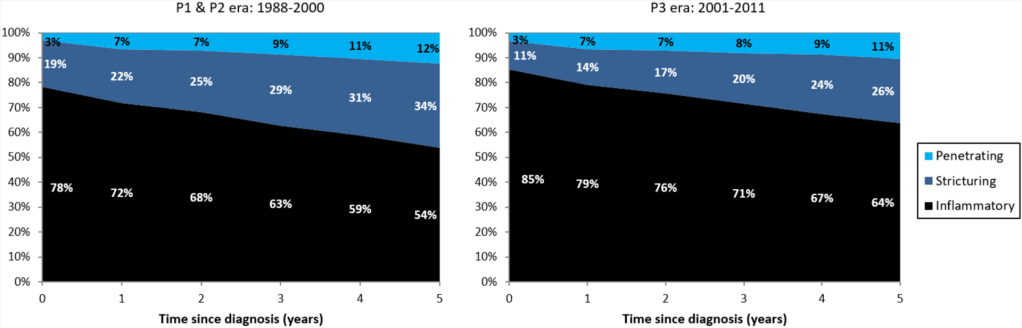
- The highest increase in CD and UC incidence rates occurred in children and young adults, respectively.
- The prevalence of IBD doubled from 1995 to 2016; the greatest increase (2.5-fold) was in UC prevalence among individuals aged >40 years. During this period, the median age of the IBD population increased by 6 to 7 years.
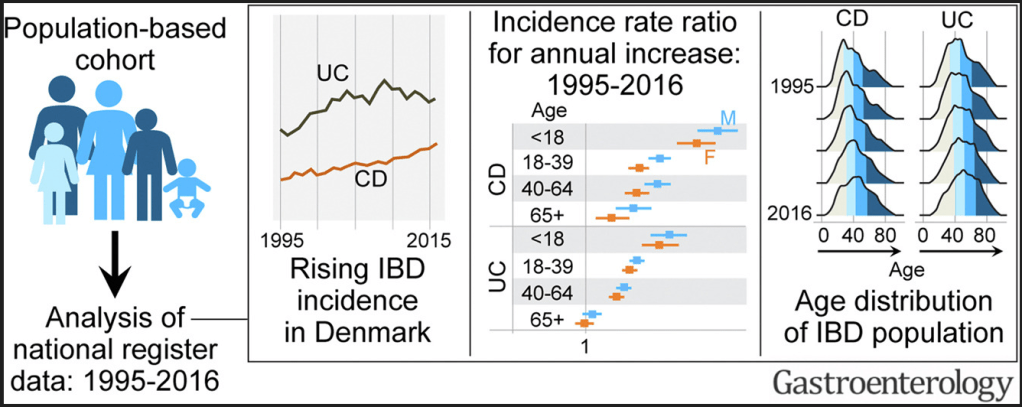
Y Pan et al. Inflamm Bowel Dis 2022; 28: 1865-1871. Utility of Therapeutic Drug Monitoring for Tumor Necrosis Factor Antagonists and Ustekinumab in Postoperative Crohn’s Disease
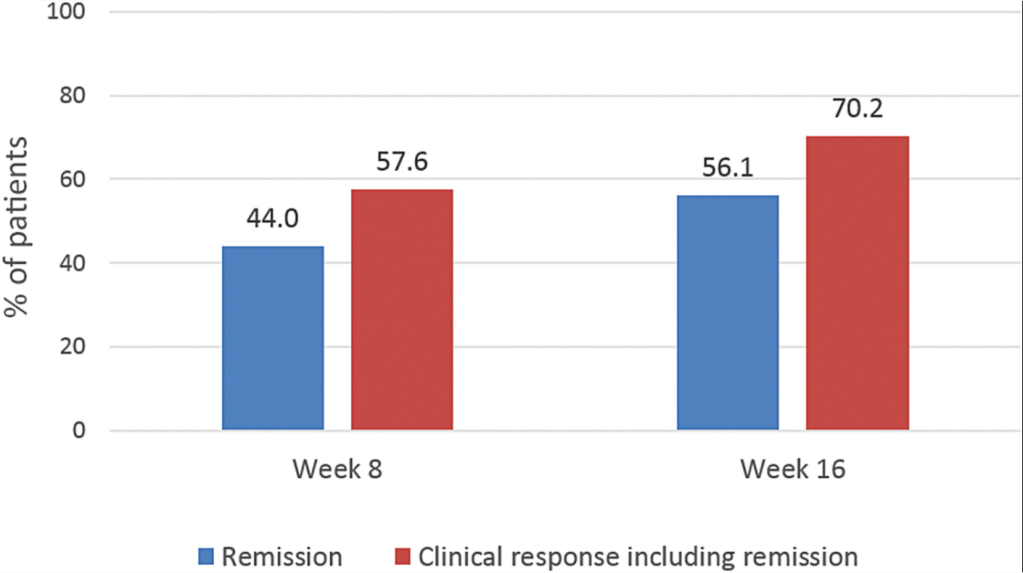
In this retrospective study (n=130), therapeutic drug levels in the postoperative period were associated with improved outcomes for anti-TNF agents (infliximab (IFX) or adalimumab (ADA) but NOT for ustekinumab (UST):
- In patients with IFX ≥3 µg/mL, higher rates of deep remission (39% vs 0%; P = .02) existed compared with those with IFX less than 3 µg/mL. This was true for clinical remission (44% vs 9%; P = .04) and objective (83% vs 62%; P = .1) remission.
- In patients with ADA ≥7.5 µg/mL, rates of deep (42% vs 0%; P = .02), clinical (42% vs 0%; P = .02), and objective (88% vs 40%; P = .007) remission were higher than patients with lower concentrations.
- For UST, rates of deep (28% vs 17%; P = 1.0), clinical (33% vs 33%; P = 1.0), and objective (70% vs 67%; P = 1.0) remission were similar between patients regardless of drug concentration.
S Sasidharan et al. Inflamm Bowel Dis 2022; 28: 1833-1837. Fecal Calprotectin Is a Predictor of Need for Rescue Therapy in Hospitalized Severe Colitis
In this retrospective study (n=147), a fecal calprotectin >800 mcg/g independently predicted the need for inpatient medical rescue therapy (odds ratio, 2.61; 95% CI, 1.12-6.12). An admission calprotectin >800 mcg/g independently predicted surgery within 3 months (odds ratio, 2.88; 95% CI, 1.01-8.17). My take: This is the least surprising study I’ve read this past month —those with more severe colitis, based on calprotectin values, were more likely to need more intensive treatments.
R Dawson et al. Inflamm Bowel Dis 2022; 28: 1859-1864. Comparing Effectiveness of a Generic Oral Nutritional Supplement With Specialized Formula in the Treatment of Active Pediatric Crohn’s Disease
In this retrospective pediatric study (n=171), the authors found that a generic oral supplement (Fortsip) was as effective as a specialized formula (Modulen IBD) for enteral nutrition. “No difference was demonstrated in remission rate (Fortisip n = 67 of 106 [63%] vs Modulen IBD n = 41 of 64 [64%], P = .89), nonadherence rate (Fortisip n = 7 of 106 [7%] vs Modulen IBD 3 of 64 [5%], P = .57) or method of administration.” The main difference in outcome was a lower expense in the group receiving the generic formula. My take: This study is in agreement with previous studies.
Related blog posts: