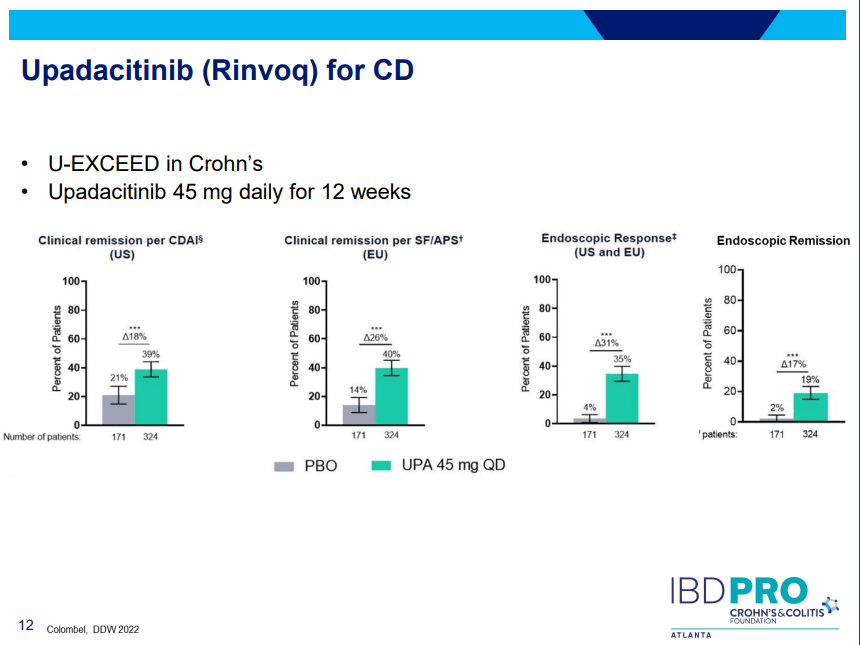
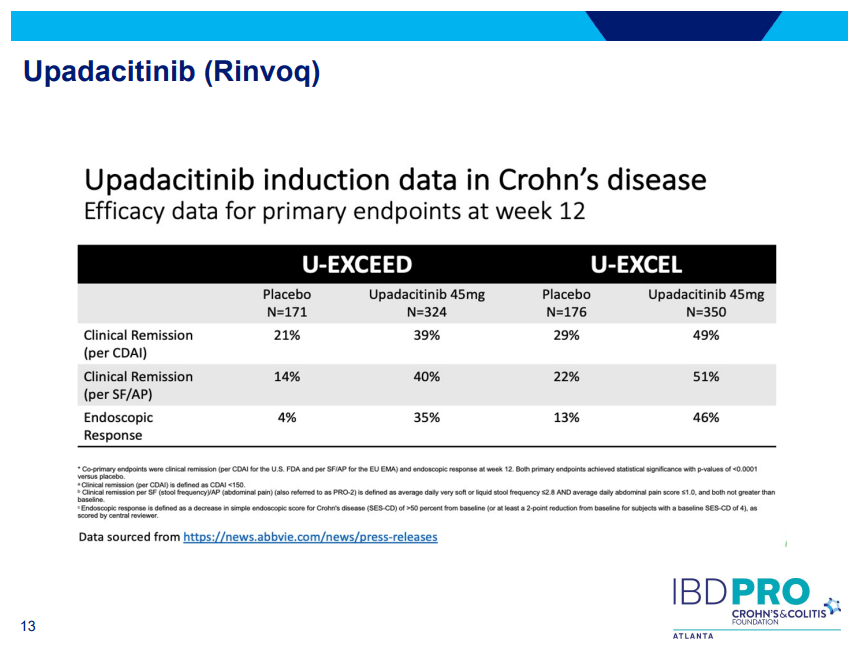
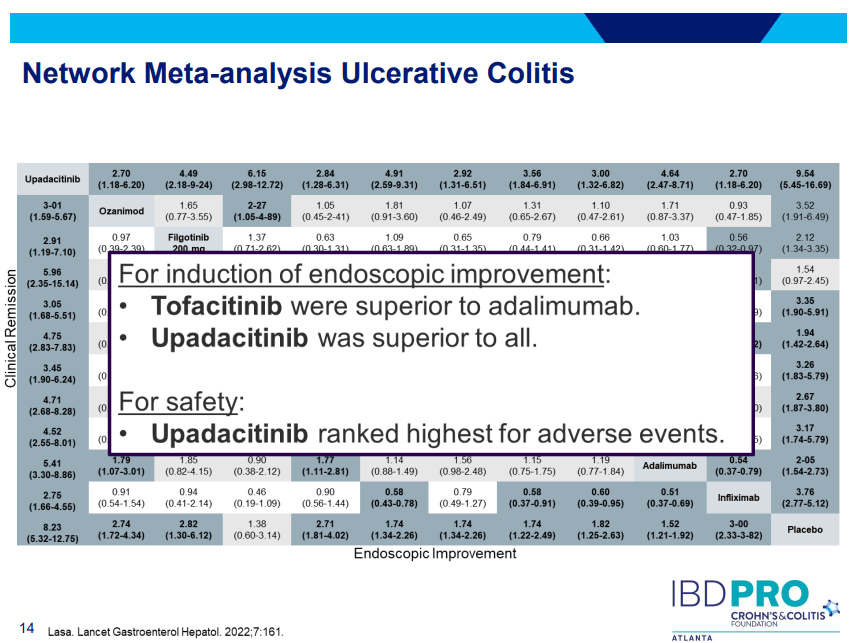
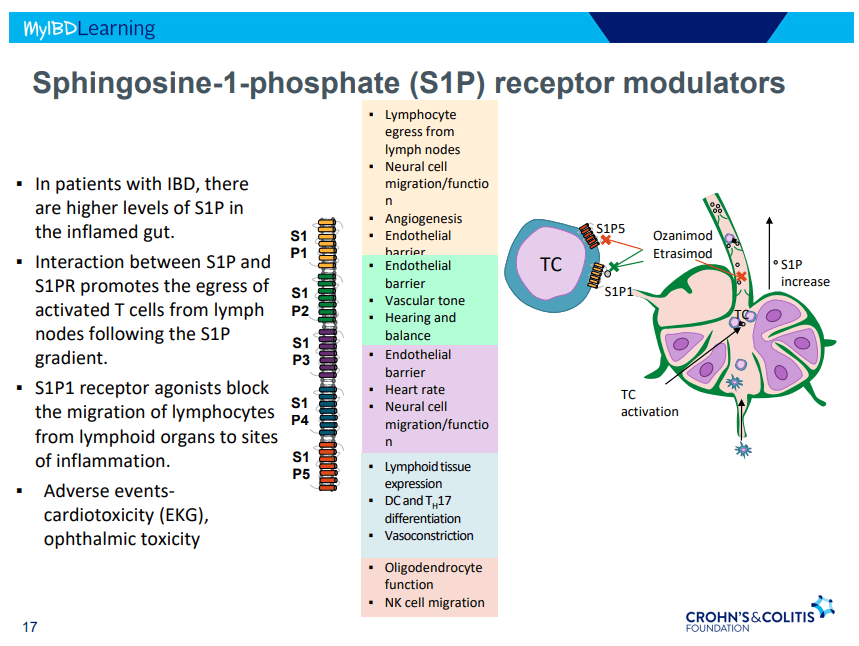
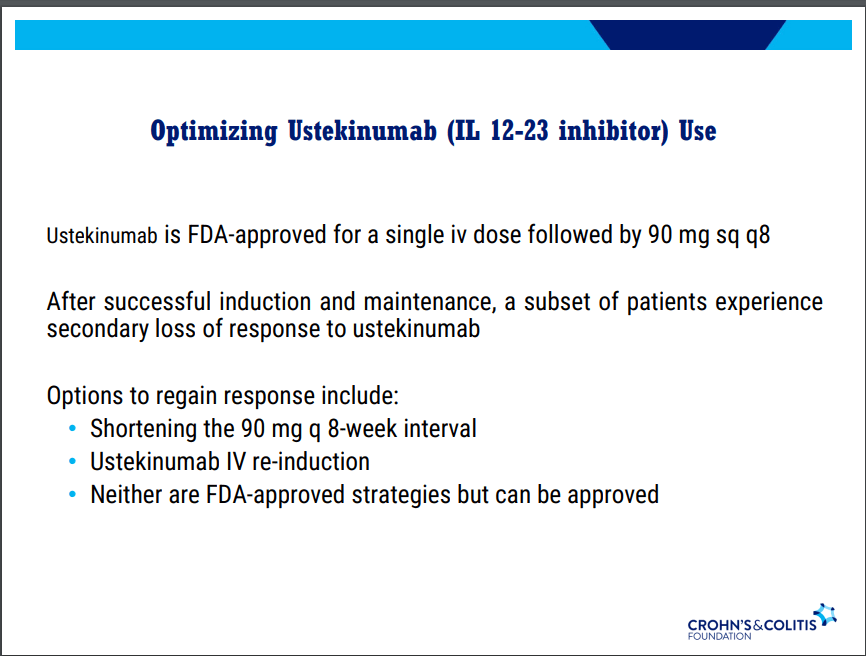
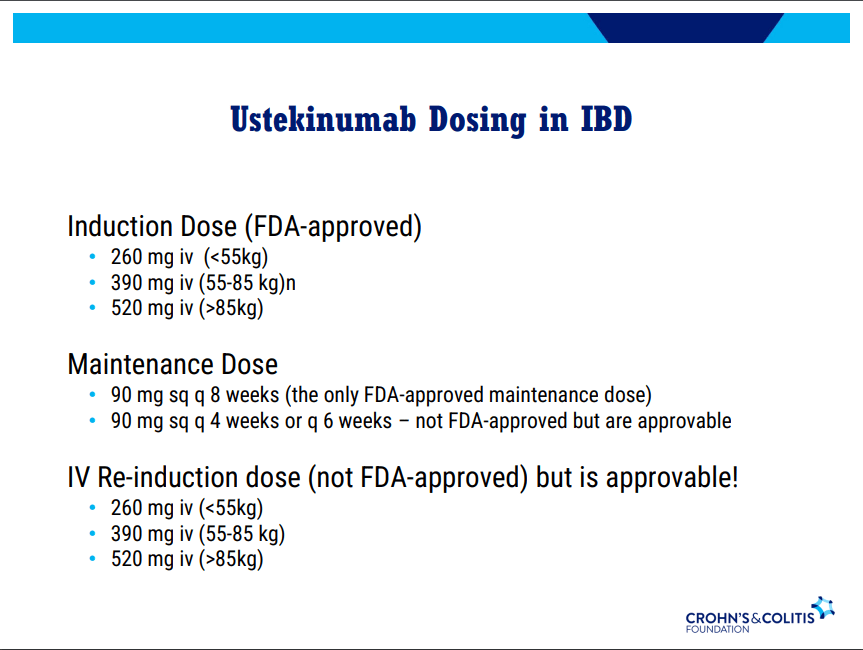
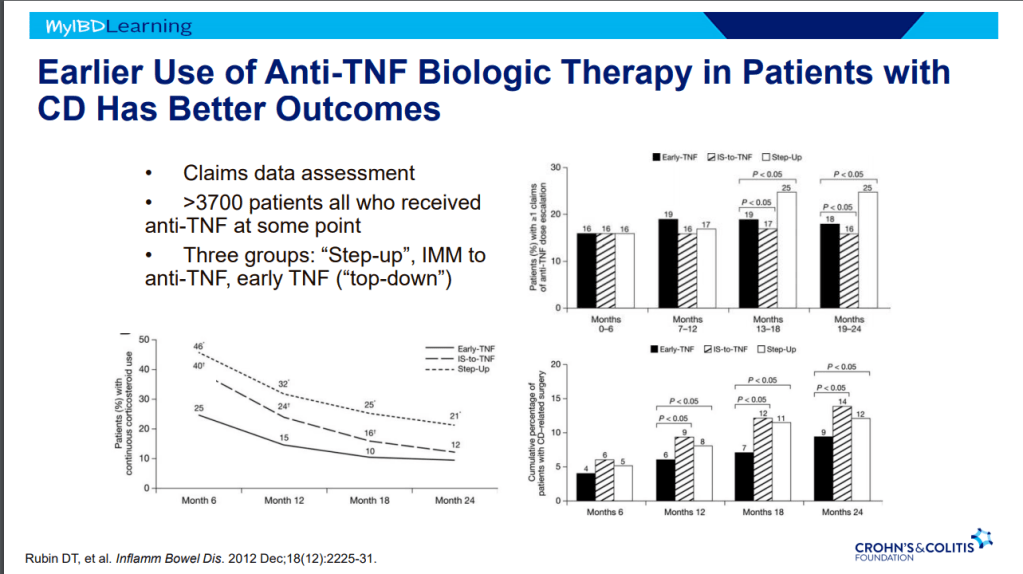
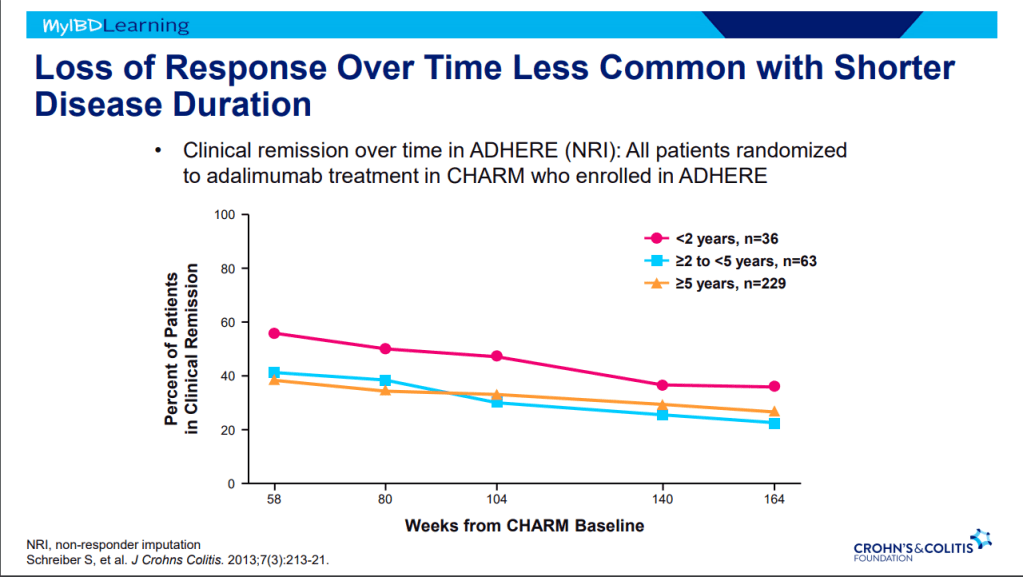
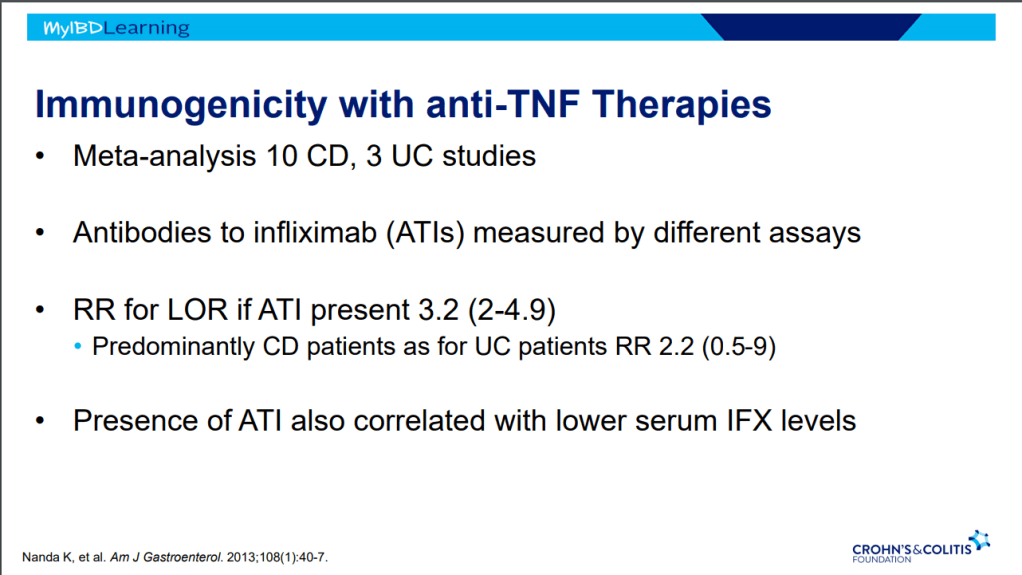
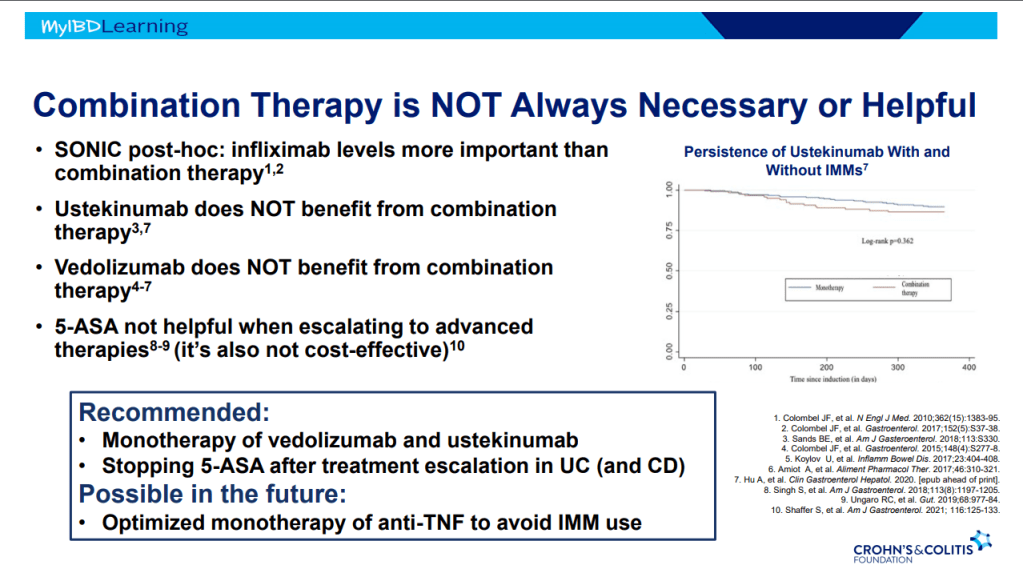
D Ahuja et al. Am J Gastroenterol 2023; Open Access! Comparative Speed of Early Symptomatic Remission With Advanced Therapies for Moderate-to-Severe Ulcerative Colitis: A Systematic Review and Network Meta-Analysis Thanks to Ben Gold for this article.
Key findings:
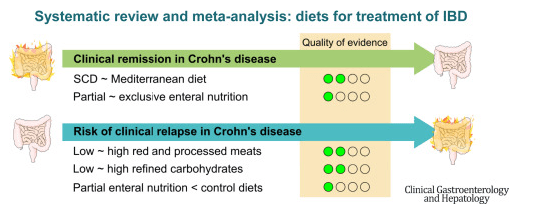
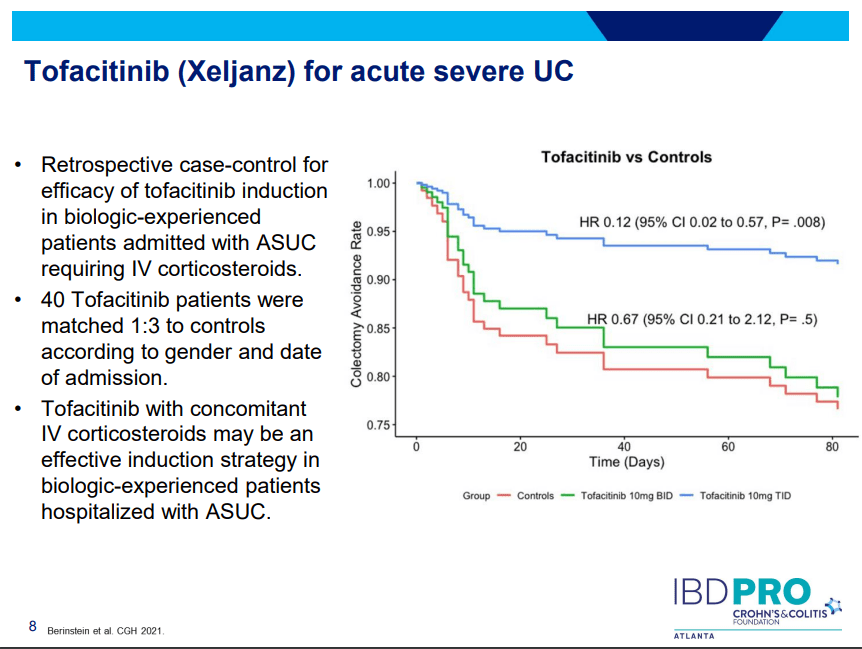
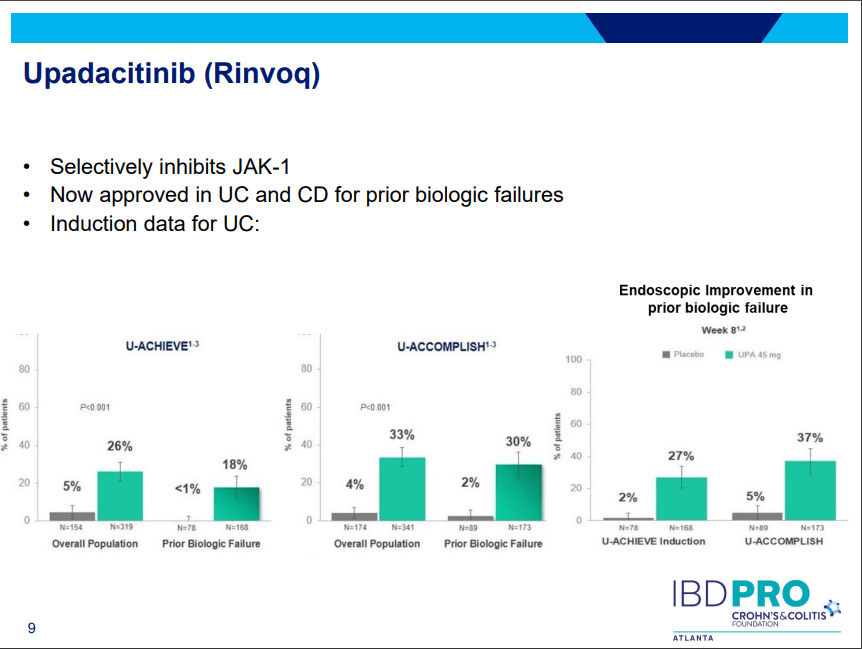
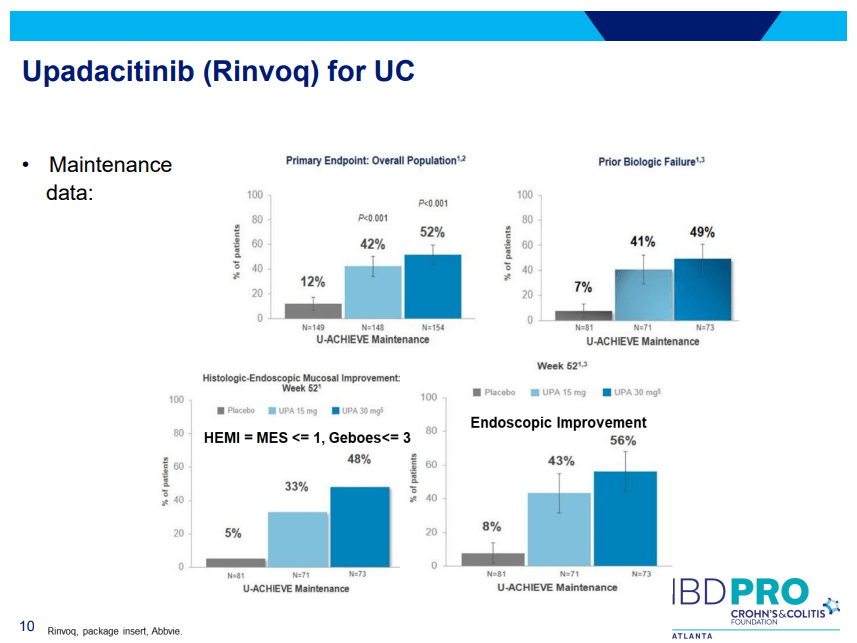
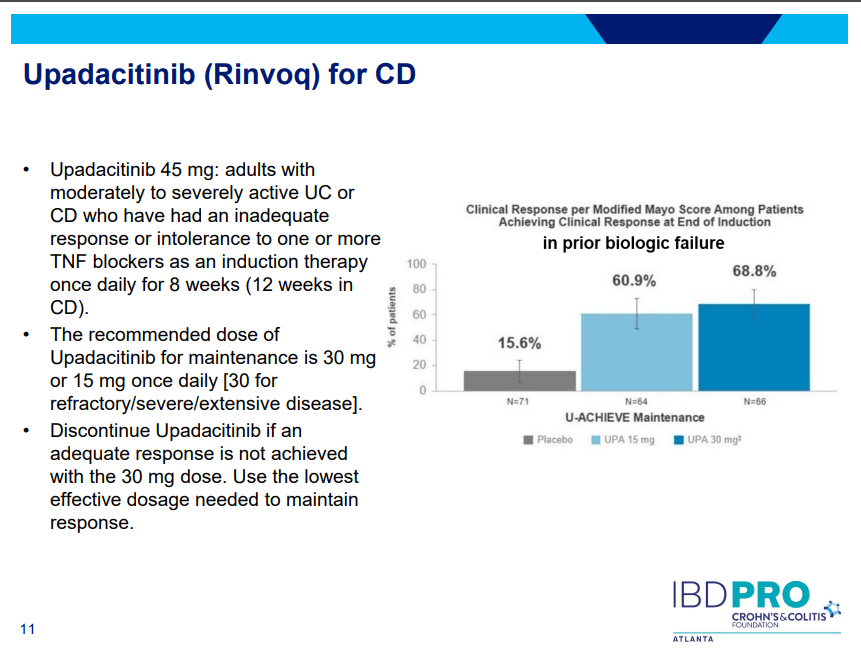
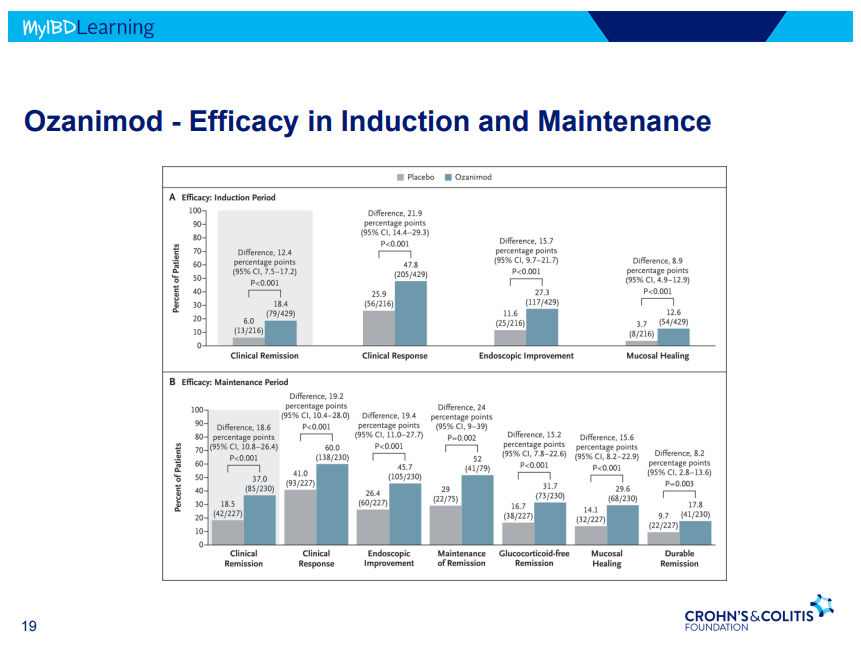
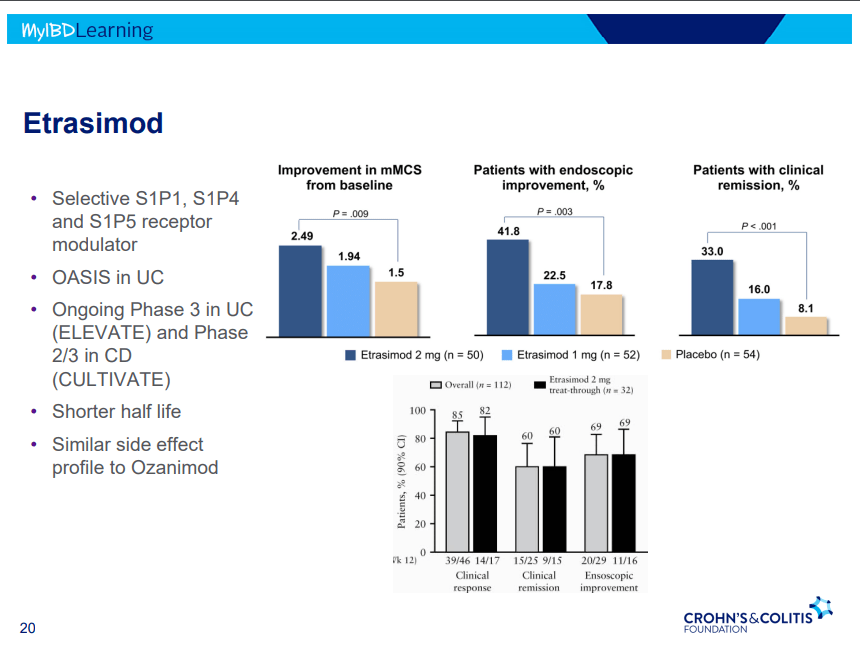
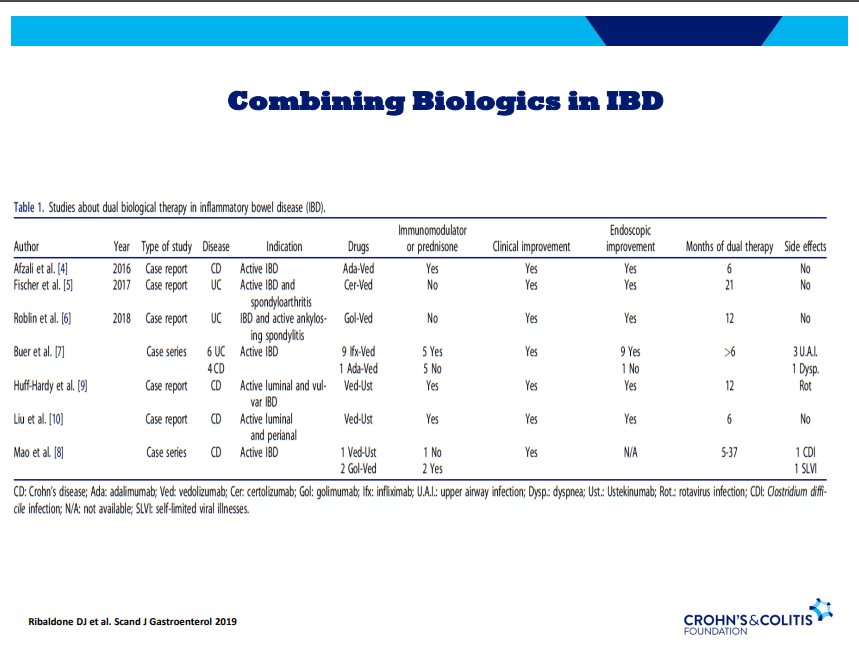
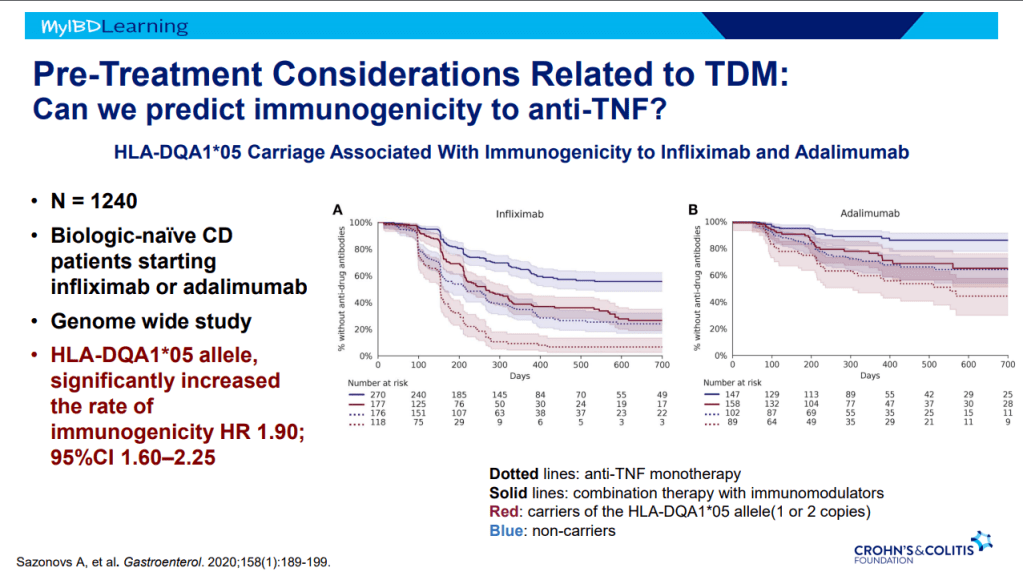
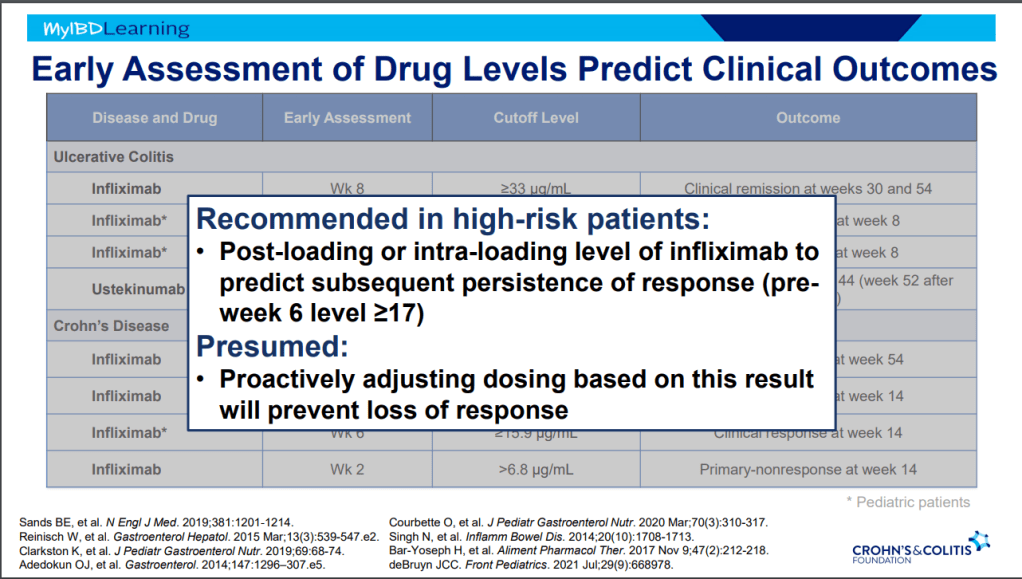
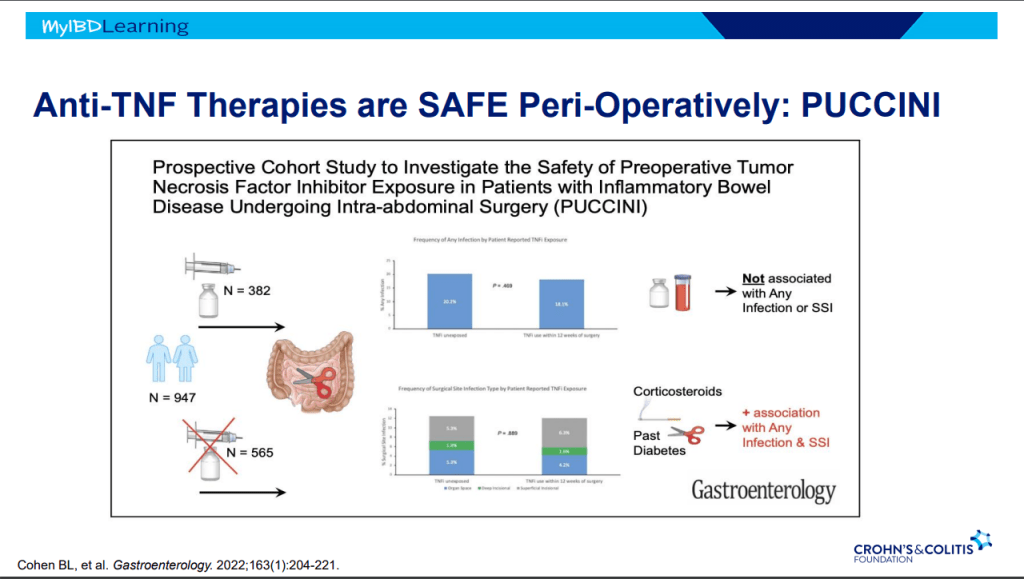
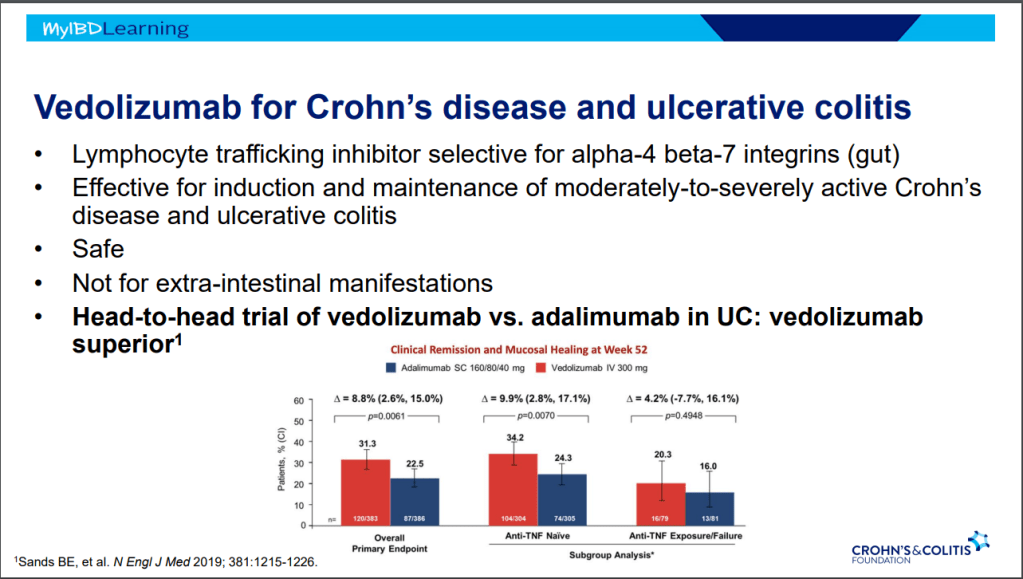
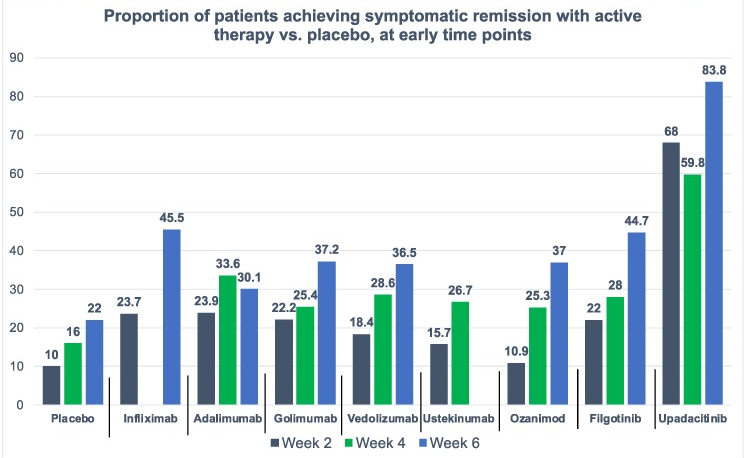
- On network meta-analysis of 14 RCTs, upadacitinib was more effective than all agents in achieving symptomatic remission at weeks 2 (range of RR, 2.85–6.27), 4 (range of RR, 1.78–2.37), and 6 (range of RR, 1.84–2.79).
This study has a number of limitations including the following:
- Potential differences in patient-level characteristics between these trials
- Symptoms may not always correlate with endoscopic findings
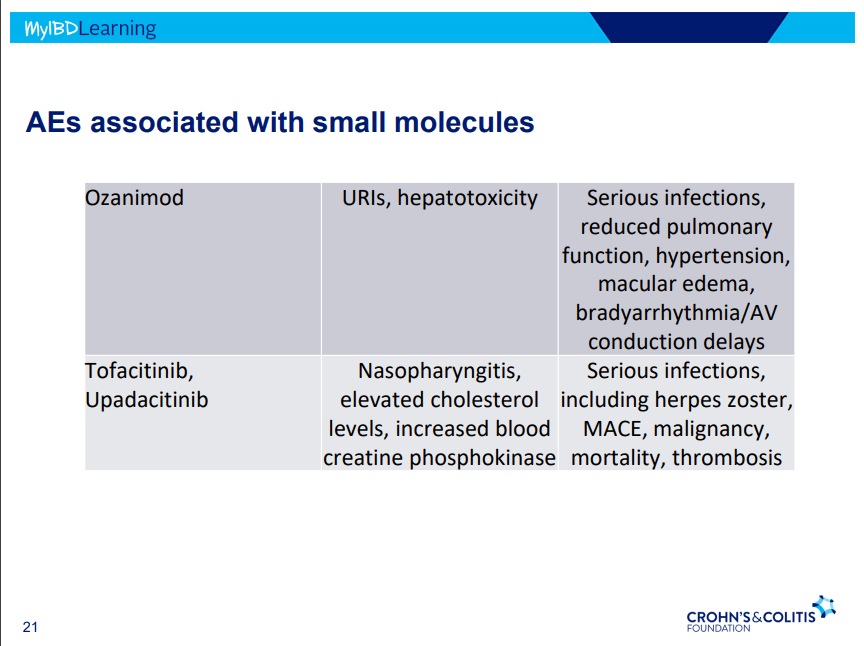
- Data from some medications (eg. tofacitinib) were incomplete and not included
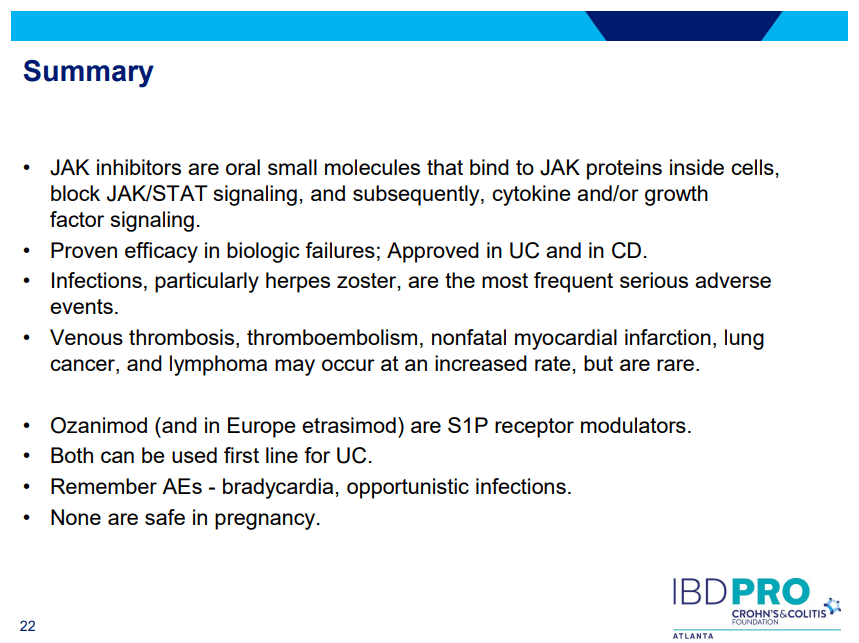
My take: This study indicates an impressive early symptomatic response to upadacitinib compared to other agents for ulcerative colitis.
Related blog posts:
- CCFA 2023 (Atlanta) Part 3
- More Data: Upadacitinib “is Effective and Safe” Plus 2 in Kids
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- New FDA Rinvoq (upadacitinib) Indication: Oral Treatment For Crohn’s
- Upadacitinib Receives FDA Approval to Treat Adults with Ulcerative Colitis
- FDA Slaps Restrictions on JAK Inhibitors Over Serious Safety Risks