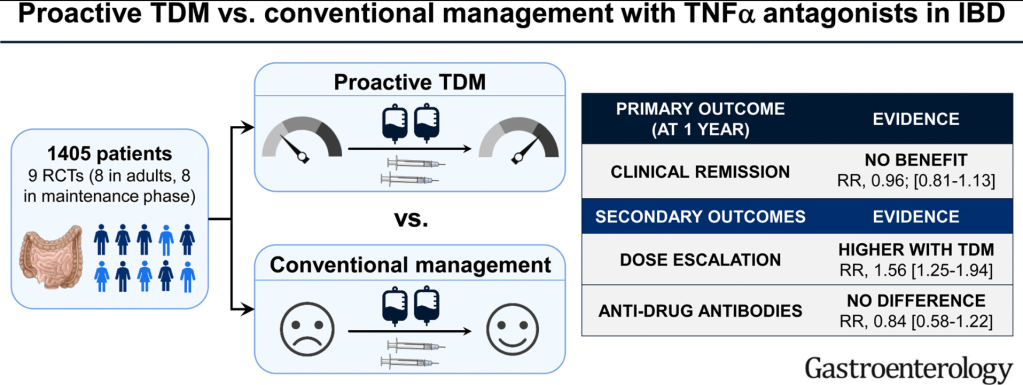
A widely covered news story in October 2022 was the disappointing results/modest benefits of a colonoscopy screening study. This study actually supports the use of colonoscopy to reduce colorectal cancer deaths but shows that typical screening programs may not work well if patients don’t show up for the test.
M Bretthauer et al. NEJM 2022; 387: 1547-1556. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death
Methods: This was “a pragmatic, randomized trial involving presumptively healthy men and women 55 to 64 years of age drawn from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. The participants were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (the invited group) or to receive no invitation or screening (the usual-care group).”
There were 84,585 participants in Poland, Norway, and Sweden — 28,220 in the invited group,
Key findings:
- Only 11,843 (42.0%) in the invited group underwent colonoscopy screening
- During a median follow-up of 10 years, 259 cases of colorectal cancer were diagnosed in the invited group as compared with 622 cases in the usual-care group
- The risk of colorectal cancer at 10 years was 0.98% in the invited group and 1.20% in the usual-care group, a risk reduction of 18%
- The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (risk ratio, 0.90; 95% CI, 0.64 to 1.16)
- The risk of death from any cause was 11.03% in the invited group and 11.04% in the usual-care group
If all invited participants had received a colonoscopy, the authors estimate the risk of colorectal cancer would have decreased from 1.22% to 0.84% and the risk of colorectal cancer death would have been reduced from 0.3% to 0.15% (a 50% drop).
My take: Colonoscopy as a screening tool only works if it is performed. Given the low response rate for screening, other tools like an annual fecal immunochemical test (FIT) need to be considered as alternatives.

Related blog posts:
- Five Ways to Lower the Risk of Colon Cancer
- 45 Years –The New Recommendation for Colorectal Cancer Screening
- Colorectal Cancer in Patients Up to Age to 25 Years
- Diet, Meat, and Colorectal Cancer
- Cancer due to Overweight/Obesity
- Better Diet, Lower Mortality
- For Increased Longevity: More Greens are Good