A landmark study (BE Sands et al. NEJM 2019; 381: 1201-14) shows that ustekinumab (Stelara) can be an effective therapy for moderate-to-severe ulcerative colitis (UC); it is already an approved, established therapy for Crohn’s disease. This randomized placebo-controlled study included an 8-week induction trial (n=961) followed by a 44-week maintenance trial (n=523) for patients with response.
Clinical remission was defined as a total socre of ≤2 on the Mayo scale (range 0-12) and no subscore >11 on any of the four Mayo scale components.
Key findings:
- During induction, there was a similar clinical remission rate between those who received 130 mg fixed intravenous dose compared to those who received 6 mg/kg: 15.6% and 15.5% compared to 5.3% for placebo group.
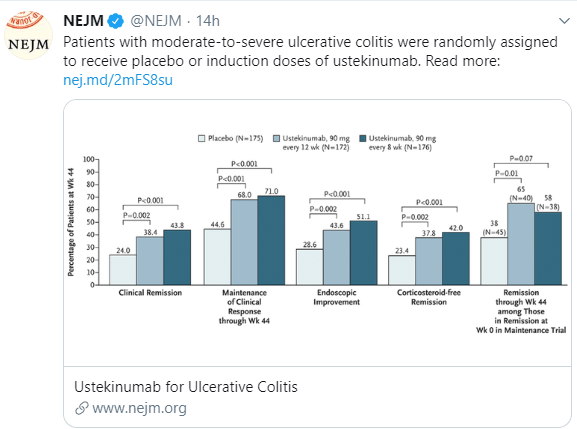
- During maintenance, among patients receiving 90 mg every 8 weeks the clinical remission rate at 44 weeks was 43.8%, in those with 90 mg every 12 weeks the rate was 38.4%; placebo group was 24.0%.
- The response to ustekinumab occurred in those with or without previous treatment failure with biologic agents, though response was lower in both induction and maintenance in those with prior treatment failure. In both phases, at least 59% of participants had failed either or both anti-TNF agents or vedolizumab.
- In this study, there were similar serious adverse events with ustekinumab compared to placebo. In the treatment groups, there were two deaths (one from ARDS, one from esophageal varices) and 7 cases of cancer (3 nonmelanoma skin cancer, two colon cancer, one prostate, one renal). There was one death from testicular cancer in the placebo group. Also four patients in the ustekinumab group had opportunistic infections including CMV in two, legionella in one and HSV in one.
In terms of dosing, the authors note that there was greater improvement in calprotectin values during induction in the group who received 6 mg/kg compared to those who received 130 mg. At week 44, using more objective and stringent end points (eg. endoscopic improvement), greater clinical benefit was observed with the every 8 week regimen.
Visual abstract from NEJM Twitter Feed:
The following image depicts patients response during the maintenance phase –the lightest color is placebo, followed by every 8 weeks, and then the darkest color is every 12 weeks. The x-axis measures (left to right) are clinical remission, maintenance of clinical response at week 44, endoscopic improvement, corticosteroid-free remission, and remission at 44 weeks in those with remission after induction.
My take: Ustekinumab is more effective for placebo in patients with ulcerative colitis. More experience is needed to understand its long-term safety.
Related blog posts:
- Landmark Publication for Ustekinumab (Stelara)
- Ustekinumab in Pediatric Clinical Practice
- More Data for Ustekinumab in Crohn’s Disease
- Predicting Response to Vedolizumab and Ustekinumab for IBD
- Therapeutic drug monitoring for ustekinumab (Stelara)
- Ustekinumab for Crohn’s Disease
- Ustekinumab: NASPGHAN17 Poster from CHOP This link has a poster (at the bottom of this post) explaining CHOP’s pediatric experience with ustekinumab (which showed a pretty limited response)