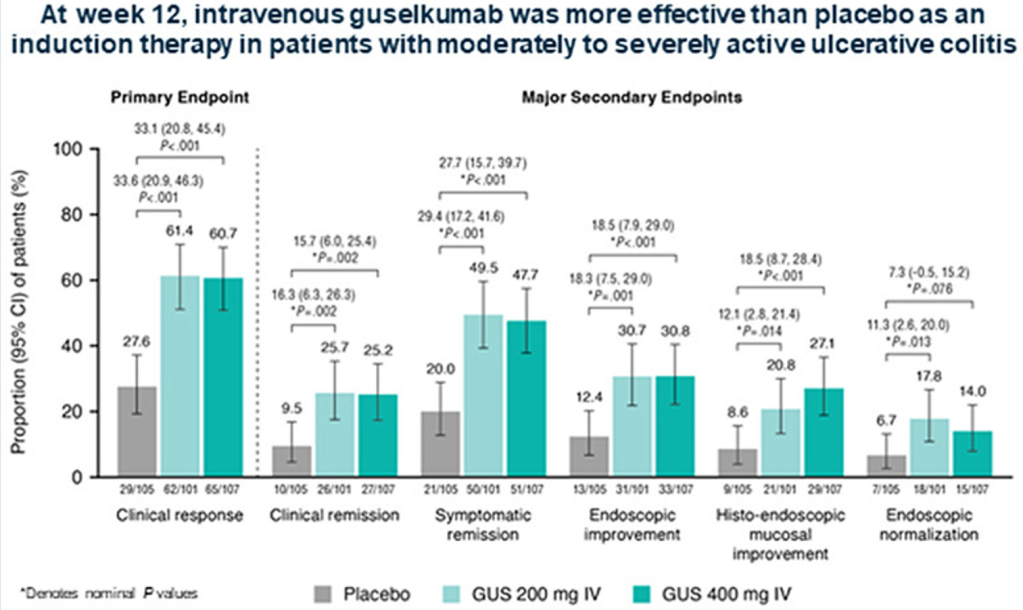
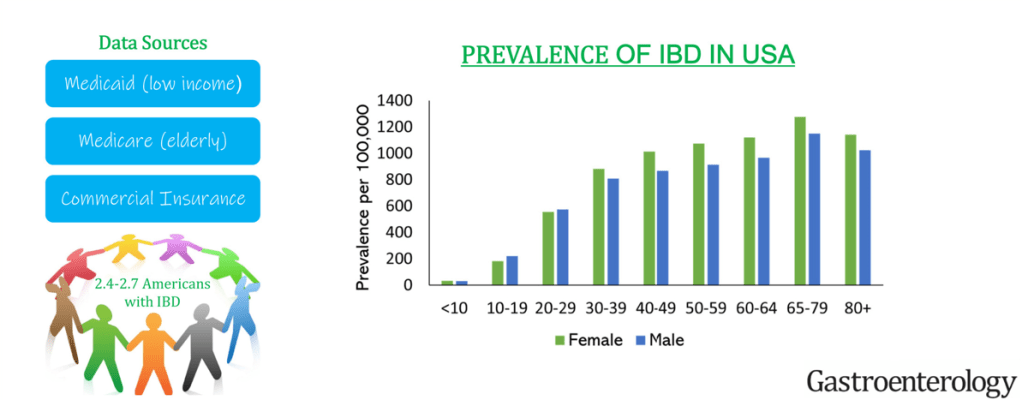
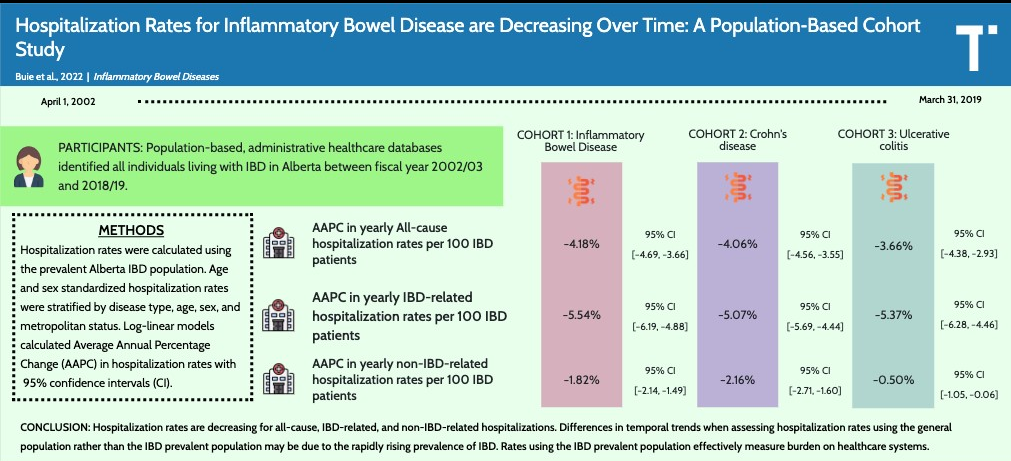
N Singh et al. Inflamm Bowel Dis 2023; 29: 1871-1878. Proton Pump Inhibitor Use Before and After a Diagnosis of Inflammatory Bowel Disease
The authors retrospectively utilized the University of Manitoba IBD Epidemiology Database includes all Manitobans diagnosed with IBD between 1984 and 2018 (n=5920). Key findings:
- Rates of PPI use in control subjects increased gradually from 1.5% to 6.5% over 15 years
- Persons with IBD had a higher rate of PPI use, peaking up to 17% within 1 year of IBD diagnosis with a rate ratio (RR) of 3.1
The authors noted an abrupt increase in PPI use within 6 months of an IBD diagnosis which could indicate that IBD-related symptoms are being mistakenly treated with a PPI or that IBD may increase reflux-related symptoms. Given the higher rate of PPI use in pre-IBD diagnosis patients, compared to controls, the authors note that “it is possible that their [PPI] use enhances the likelihood of an IBD diagnosis by their role in altering the gut microbiota.” In addition, they note that “a case-control study found that PPIs were associated with an increased risk of pediatric IBD” (NR Schwartz et al. J Pediatr Pharmacol Ther 2019; 24: 489-496).
My take: PPIs are being used more frequently. Whether PPIs are detrimental before or after a diagnosis with IBD is not clear.