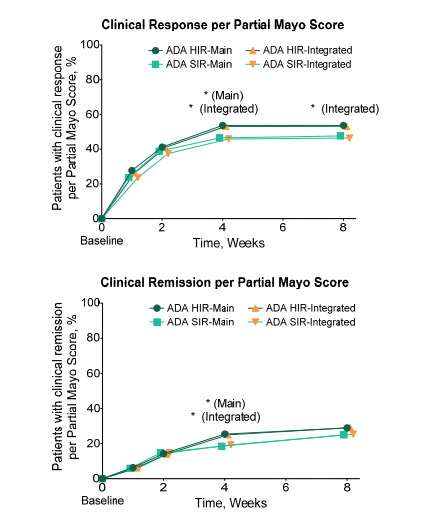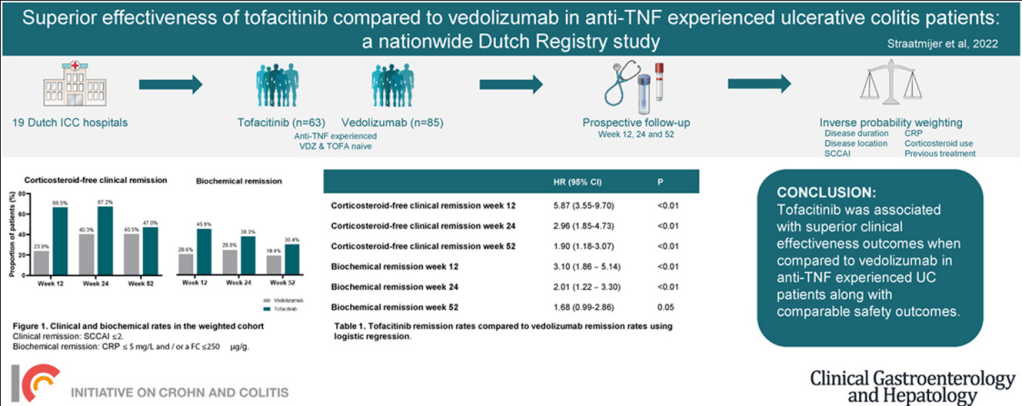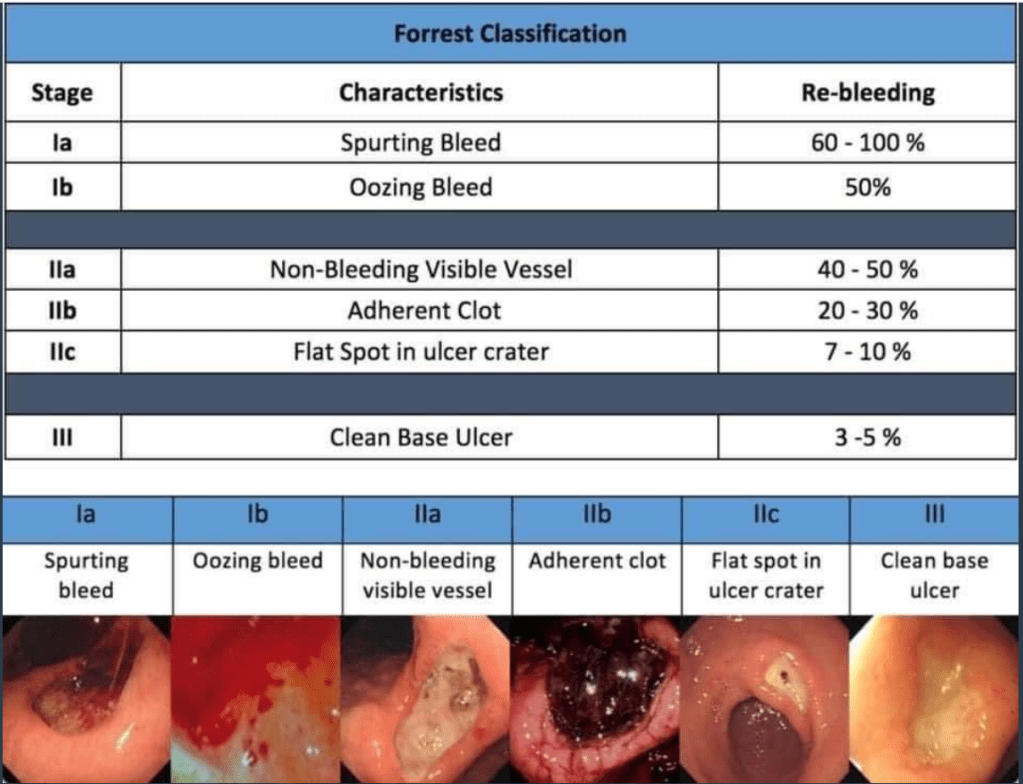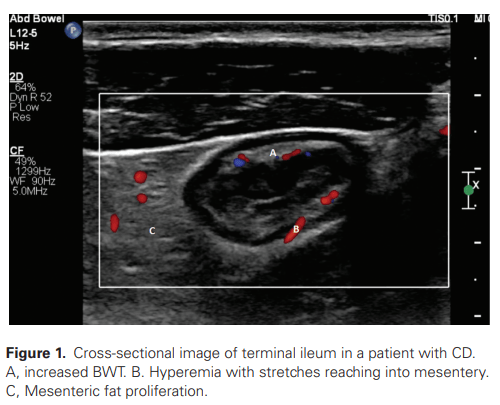
A Lembo, S Sultan et al. Gastroenterol 2022; 162: 137-151. Open access PDF: AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea

LChang, S Sultan et al. Gastroenterol 2022; 162: 118-136. Open access: AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation

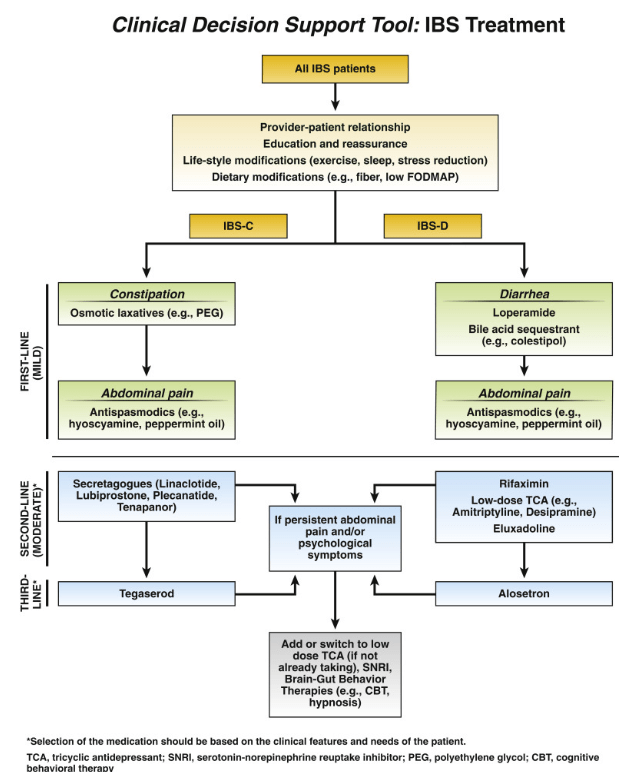
The associated 1-page summary (“Spotlight: IBS Treatment“) on pg 153 reviews society guidelines on testing in IBS. This includes for IBS-D celiac serology, calprotectin/lactoferrin, CRP, possilby Giardia antigen (if in endemic area) and possibly bile acid diarrhea testing. Not recommended include food allergy/sensitivity testing, colonoscopy if <45 years and lactulose or glucose hydrogen breath testing. This 1-page summary details therapeutic dosing and costs. Monthly costs of selected medications according to this report:
- Lubiprostone (Amitiza): $374
- Linaclotide (Linzess): $523
- Pleacnatide (Trulance): $528
- Tegaserod (Zelnorm): $480
- Tenapanor (IBSRELA): $1680
- Rifaximin (Xifaxan) $1544 (for 14 day course)
- Eluxadoline (Viberzi): $1550
- Alosetron (Lotronex): $1457-1929 (starting dose), $2915-3859 (max dose)
My take: These guideline publications provide comprehensive information regarding potential pharmacological therapies.
Related blog posts:
- ACG IBS Clinical Guidelines
- Low Quality Evidence for IBS Dietary Therapy
- Carlo DiLorenzo: Lessons Learnt Over 30 Years
- “Implementing psychological therapies for gastrointestinal disorders in pediatrics”
- Course of Functional Abdominal Pain Before and During Pandemic
- Can We Predict Which Patients With Irritable Bowel Will Respond to Dietary Manipulation Based on Their Microbiome?
- IBS Part 1 2017
- An Unexpected Twist for “Gluten Sensitivity” | gutsandgrowth
- Prospective Pediatric Study of the Persistence and Progression of Recurrent Abdominal Pain | gutsandgrowth