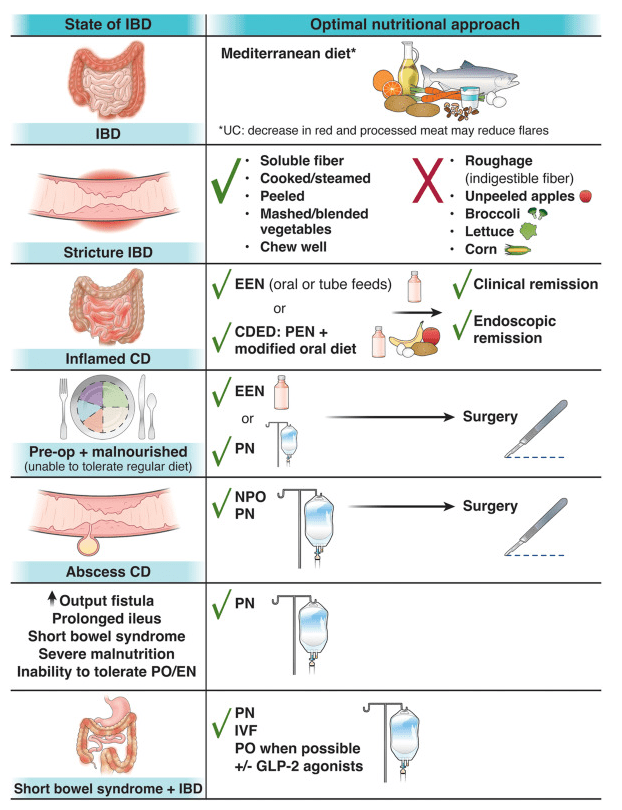
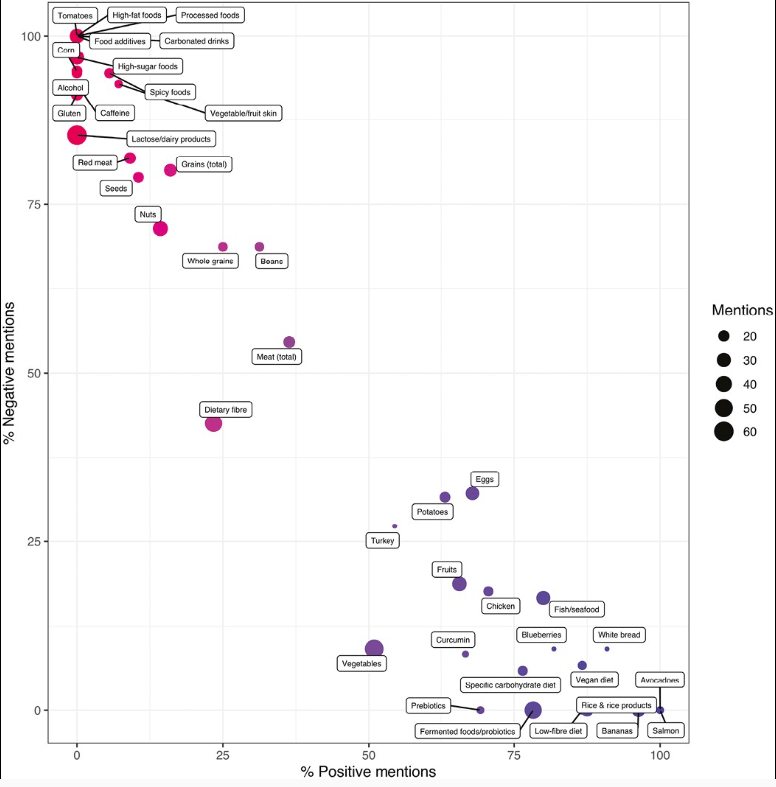
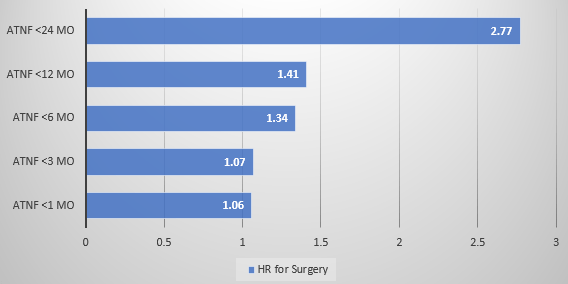
Dr. Joel Rosh gave our group an excellent update on sequencing therapy for ulcerative colitis (UC). My notes below may contain errors in transcription and in omission. Along with my notes, I have included many of his slides.

- There are only two FDA-approved biologics in pediatric Ulcerative Colitis. It typically takes 8-10 years for a medication with approval in adults to receive FDA approval in children

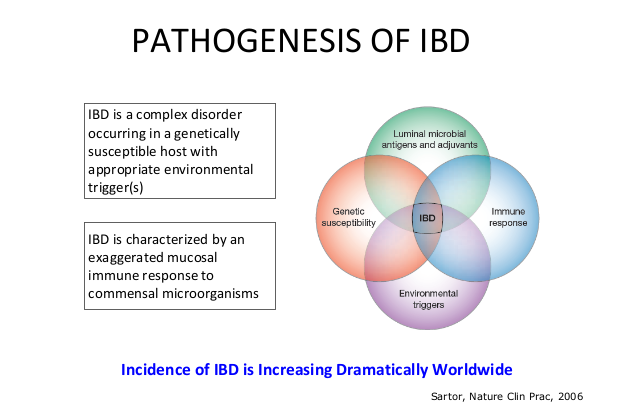
- The concept of IBD as two diseases, Crohn’s disease and UC, is flawed; there are more than 200 susceptibility genes for inflammatory bowel disease
- There has been an increasing incidence and prevalence of IBD. Some of this increase is likely due to our diet and its effects on the microbiome
- Ultrasound is a nice tool to see what is going on in real time and shows that UC is really a transmural disease. UC changes in the bowel can result in fibrosis

- Consider cytokine-basis for disease as a way to conceptualize disease presentation compared to organ-based disease. Many autoimmune diseases (eg. JIA, RA, Psoriasis) are different manifestations related to cytokine-based autoimmunity





- Almost all pediatric IBD can be considered higher risk based on known risk factors including disease extent (>80% of pediatric UC is pancolitis) and disease age of onset
- Mesalamine steroid-free clinical remission rates are about 1/3rd after 1 year of treatment
- Overall, there has been an improvement in colectomy rates since 2001; there still appears to be a bump in the colectomy rate after having UC for more than 10 years
- Elevated CRP is less common in patients with UC, compared to Crohn’s disease, and is a marker for more severe disease activity

- Dr. Rosh prefers to avoid some terms including biologic-naive and steroid failure; he favors biologic-unexposed for the former. For the latter, he tries to make it clear that the patient was not a steroid failure. Steroids failed the patient rather than the patient failing the steroids

- Therapeutic drug monitoring (TDM) is mainly beneficial for anti-TNF agents at this time. Use of TDM can help monotherapy achieve similar results as combination therapy. For infliximab, Dr. Rosh’s ‘rule of thumb’ is 28-18-8 for 2 week trough, 6 week trough, and maintenance trough. Therapeutic levels will meet or exceed these trough levels.
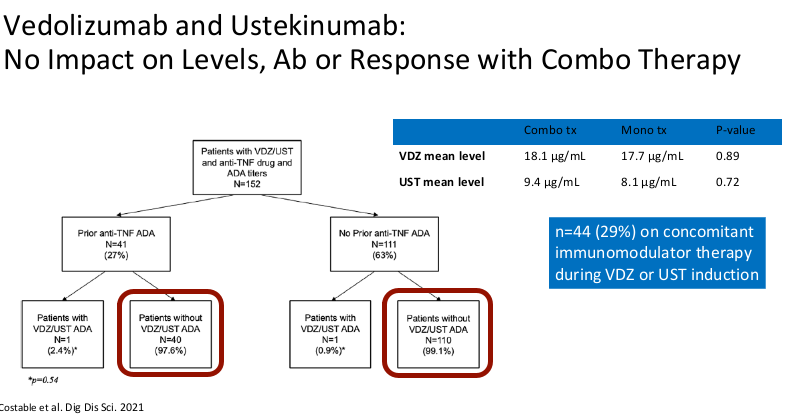
- Combination therapy has not been shown to improve pharmacokinetics for vedolizumab or ustekinumab

- Generally, a washout period is not needed when changing biologic therapies. In fact, having some overlap in the medications may have some therapeutic benefit
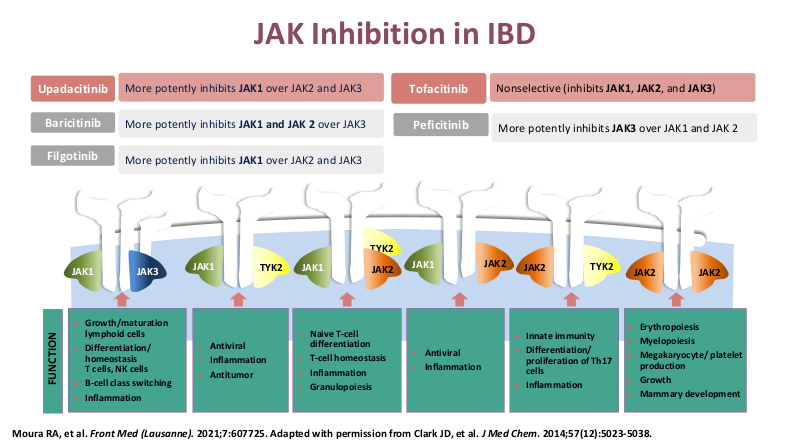


- Upadacitinib (Rinvoq) appears to be the most effective JAK for IBD. It is labelled for use as a 2nd-line agent but may be superior for some sicker patients. Rinvoq could be considered as a ‘bridge’ medication in patients with acute severe ulcerative colitis with transition to another biologic like vedolizumab
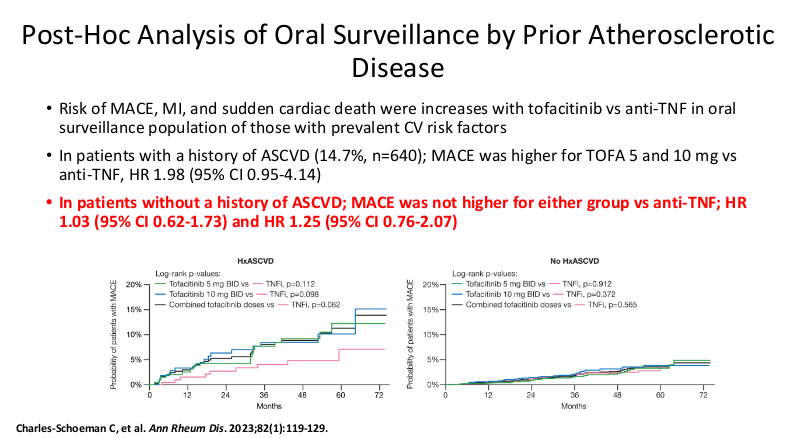
- It is important for families to be informed that there is a black box warning for the use of JAK inhibitors. However, major cardiac adverse events (MACE) do not appear to be increased in patients without preexisting cardiac disease risk factors

- “Safety Pyramid” provides some insight into biologic safety but caution is needed given the absence of adequate long-term data for many agents (Related post: IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid)
- There is an unclear role for selective sphingosine-1-phosphate receptor modulator in pediatric UC
- Vedolizumab is a good 1st line agent for pediatric UC -gut-specific, and probably the safest IBD biologic. The VARSITY trial is useful to convince insurers for approval. (Related blog posts: Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial, Vedolizumab More Effective Than Adalimumab for Ulcerative Colitis)
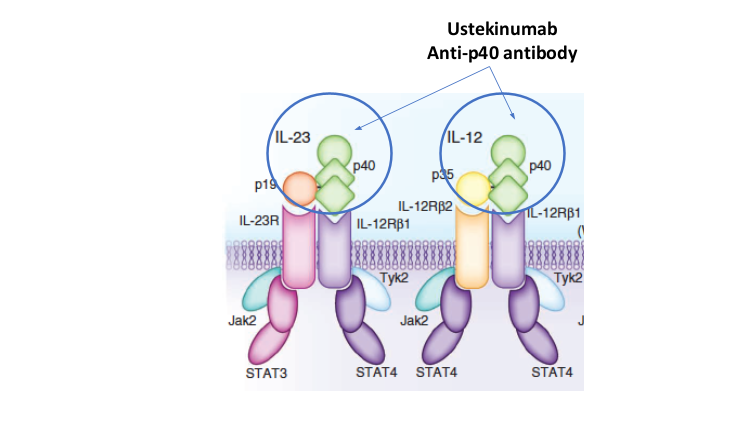

- Risankizumab appears to be more efficacious than ustekinumab in patients with IBD (Related blog post: Impressive Results for Risankizumab in Refractory Crohn’s Disease)



Related blog posts:
- IBD Updates: Understanding Newest IBD Therapies for Kids- Bowel Sounds with Joel Rosh, Hispanic Patients with IBD, More on Intestinal Ultrasound
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
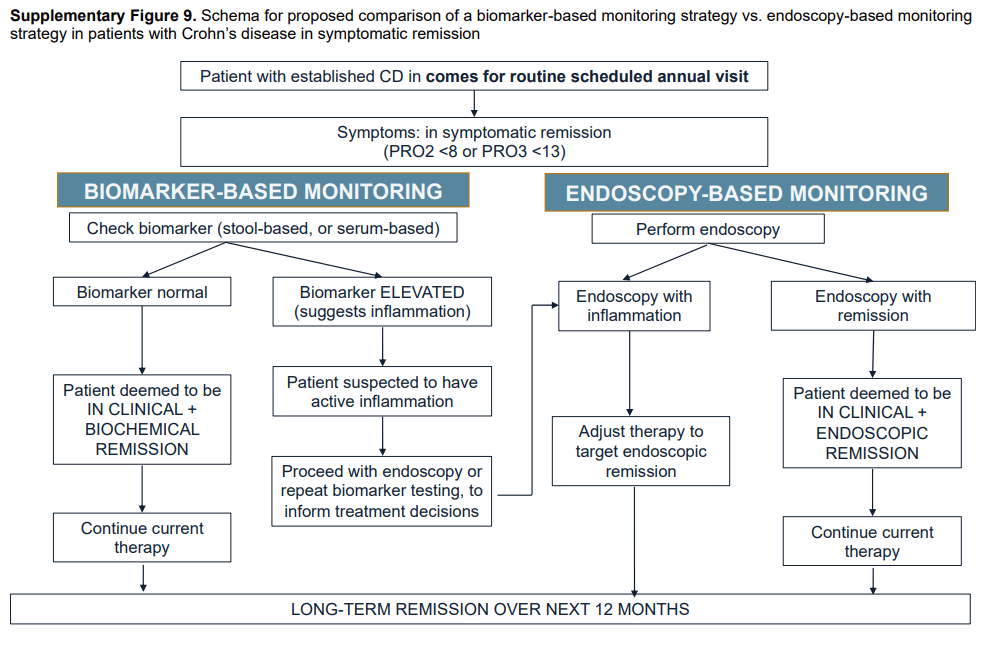
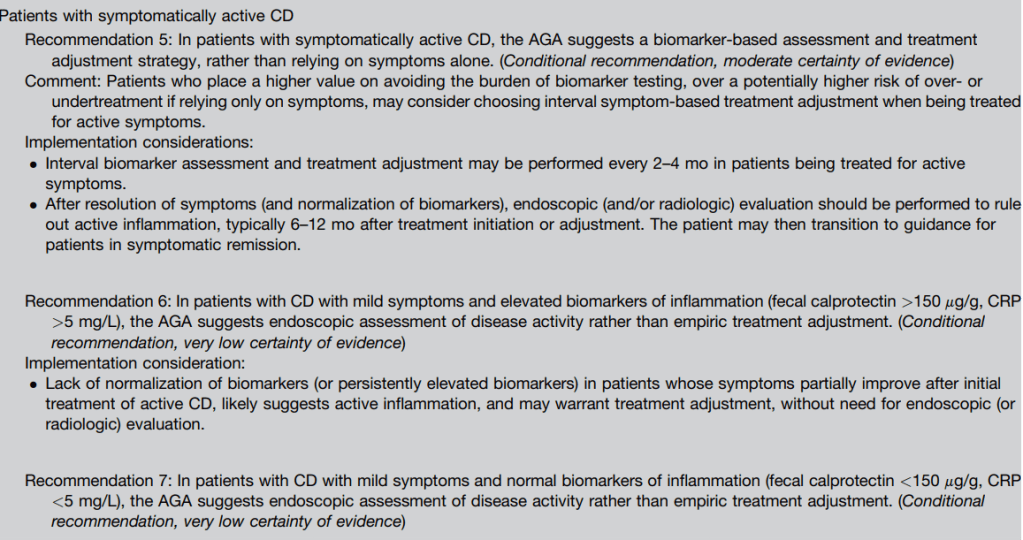
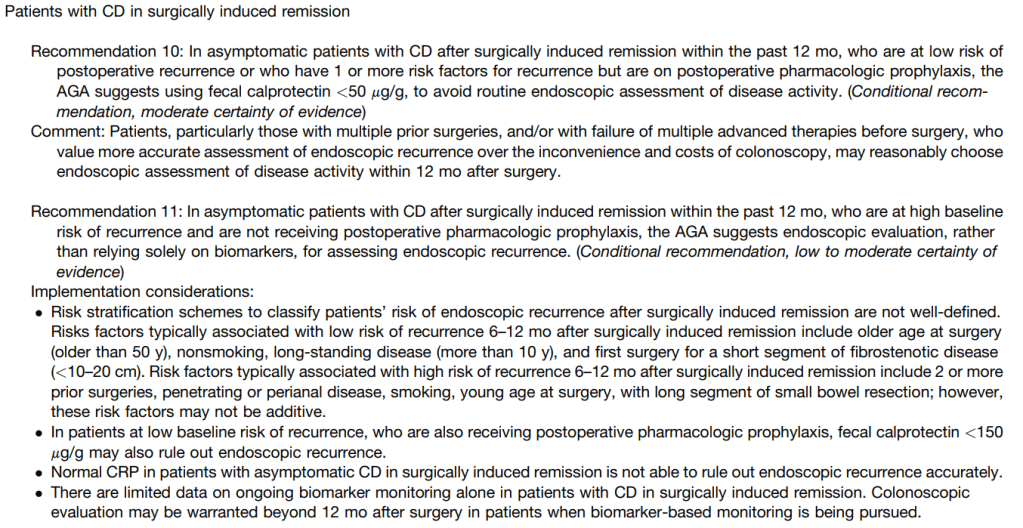
- Guideline: Biomarker Use for Ulcerative Colitis
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- Tofacitinib Outperformed Vedolizumab in Anti-TNF-experienced Ulcerative Colitis
- FDA Slaps Restrictions on JAK Inhibitors Over Serious Safety Risks
- Treatments for “Bad” Inflammatory Bowel Disease (Part 2) & Reassuring Data on Tofacitinib
- Upadacitinib Works Quickly and with High Response
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 4
- Upadacitinib Receives FDA Approval to Treat Adults with Ulcerative Colitis
- More Data: Upadacitinib “is Effective and Safe” Plus 2 in Kids
- IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid
- Dr. YouTube for IBD Dietary Advice
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.