A Buisson et al. Clin Gastroenterol Hepatol 2023; 21: 2338-2346. Open Access! Effectiveness of Switching From Intravenous to Subcutaneous Infliximab in Patients With Inflammatory Bowel Diseases: the REMSWITCH Study
In this study, 133 ot 184 patients in clinical remission agreed to switch to subcutaneous infliximab. Key findings:
- At visit 3, a relapse occurred in 10.2% (n = 6 of 59), 7.3% (n = 3 of 38), 16.7% (n = 3 of 18), and 66.7% (n = 10 of 15) (P < .001) of patients receiving 5 mg/kg every 8 weeks (5Q8W), 10Q8W, 10Q6W, and 10Q4W, respectively.
- Dose escalation to 240 mg every other week led to recapture clinical remission in 93.3% (n = 14 of 15).
- Infliximab serum levels increased after the switch (P < .0001) except for patients receiving 10 mg/kg every 4 weeks.
- Conclusion (borrowed from authors): Switching from intravenous to subcutaneous infliximab 120 mg every other week is safe and well accepted, leading to a low risk of relapse in IBD patients except for those receiving 10Q4W; these patients likely require 240 mg every other week
EV Loftus et al. Clin Gastroenterol Hepatol 2023; 21: 2193. Open Access! Therapeutic Drug Monitoring for Subcutaneous Infliximab? Too Early to Conclude (Editorial) This editorial provides a terrific analysis of the above-mentioned study. A few of the points:
- Reduced (41.7%) or stable (36.8%) serum levels of IFX after the switch (difference: V1-V0) were associated with higher risk of relapse than increased serum levels (>1 μg/mL; 12.7%; P = .020 and P = .019, respectively)
- Patients receiving IV infusion of IFX 10Q4W had a higher risk of relapse (odds ratio, 12.4; P = .017). In addition to having significantly higher serum levels than in other IFX IV regimens, this group of patients did not see a rise in IFX concentrations at V1, in contrast to other IFX regimens.
- Being overweight increases the clearance of CT-P13 SC, with an increase in clearance of 43.2% for a weight increase from 70 to 120 kg. The presence of antibodies to IFX also increases clearance by 39%. Finally, a decrease in serum albumin level (42 g/L vs 3.2 g/L) increases the clearance by 30.1%.
My take:
- Monitoring IFX levels would be helpful in patients switching from IV to SC administration, especially in higher risk groups (eg. high baseline dosing, positive anti-drug antibodies, low serum albumin, overweight individuals)
- My experience with SC biologics has been that there is a much higher rate of non-adherence than with IV infusions. If/when SC biologics are used more often, I will need to implement more intensive followup to assure patients receive both the needed medication and the needed monitoring.
Related blog posts:
- Infliximab Injections Coming Soon
- SC Option for Infliximab
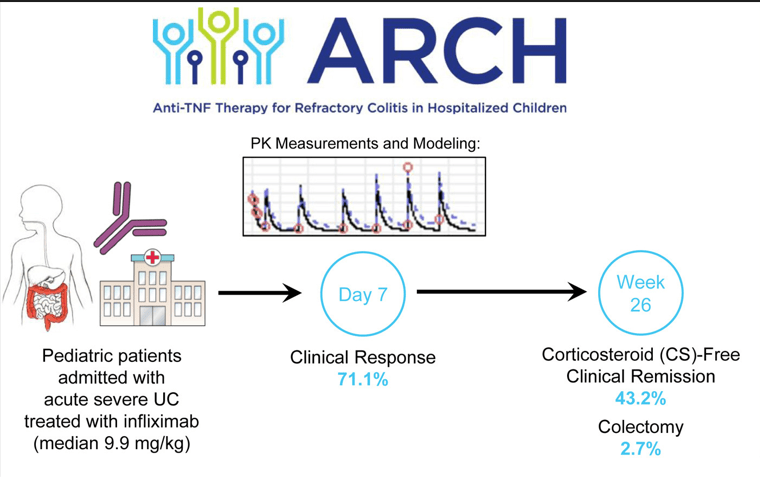
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
- Another Study Justifying Higher Infliximab Dosing in Pediatrics