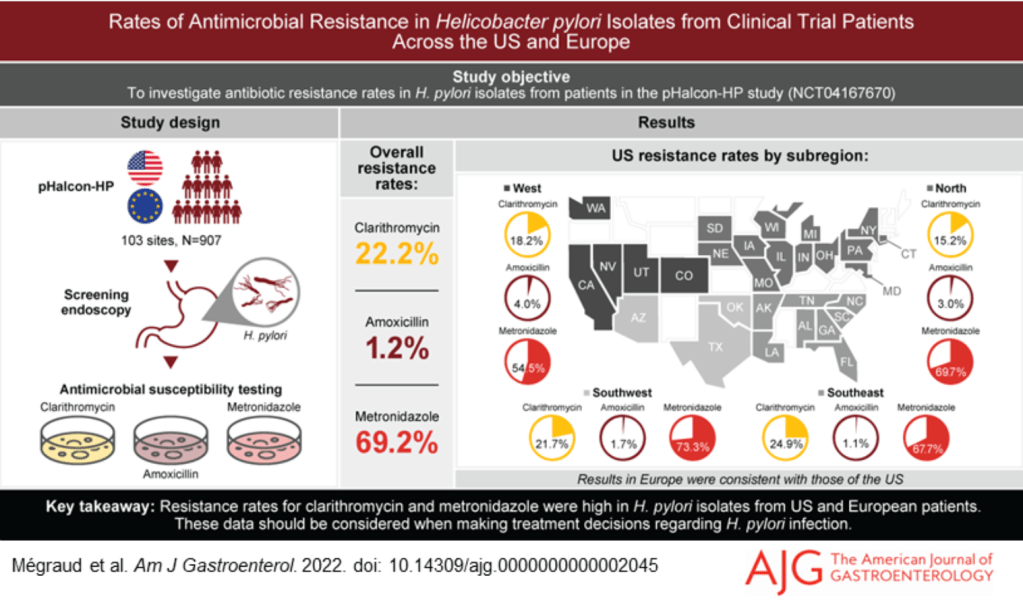
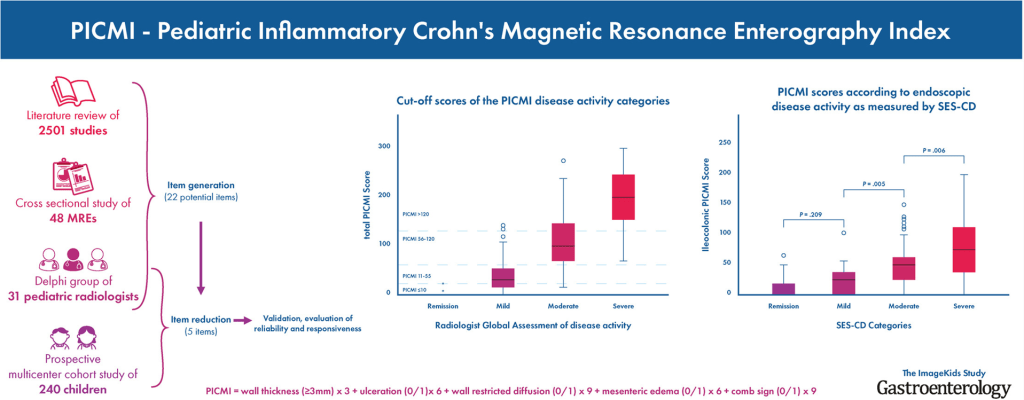
Z Wang et al. Clin Gastroenterol Hepatol 2023; 3188-3190. Therapeutic Drug Monitoring Can Guide the Intravenous-to-Subcutaneous Switch of Infliximab and Vedolizumab: A Simulation Study
The authors performed population pharmacokinetic (popPK) simulations to determine optimal dosing recommendations.
Key points:
- Infliximab: “The Q2W SC dosing regimen of infliximab has been selected with the purpose of exceeding a C,trough,ss of 5 mg/L.” This tends to align with 5 mg/kg Q8W IV dosing.
- Infliximab: “Patients on Q6W or Q8W IV infliximab can safely switch to Q2W SC infliximab…only patients on Q4W IV infliximab need Q1W SC dosing”
- Vedolizumab: “Only patients on Q4W IV vedolizumab should switch to Q1W SC dosing”
- Both agents: “Switching 4 instead of 8 weeks after the last IV dose can hit SS[steady state] faster, thereby avoiding the risk of temporary underexposure.”
My take: It is still important to see how switching from IV to SC route affects clinical outcomes in real-world cohorts. This study, though, does provide a good starting point when trying to provide the right dose frequency to achieve good therapeutic troughs.
Related blog posts:
- REMSWITCH: Infliximab IV to SC Study
- SC Option for Infliximab
- Subcutaneous Vedolizumab Now FDA-Approved for Adults with Ulcerative Colitis
- IBD Updates: SC Vedolizumab, PRODUCE study: Specific Carbohydrate Diet, Racial Epidemiology of IBD, and Microbiome in UC

Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.