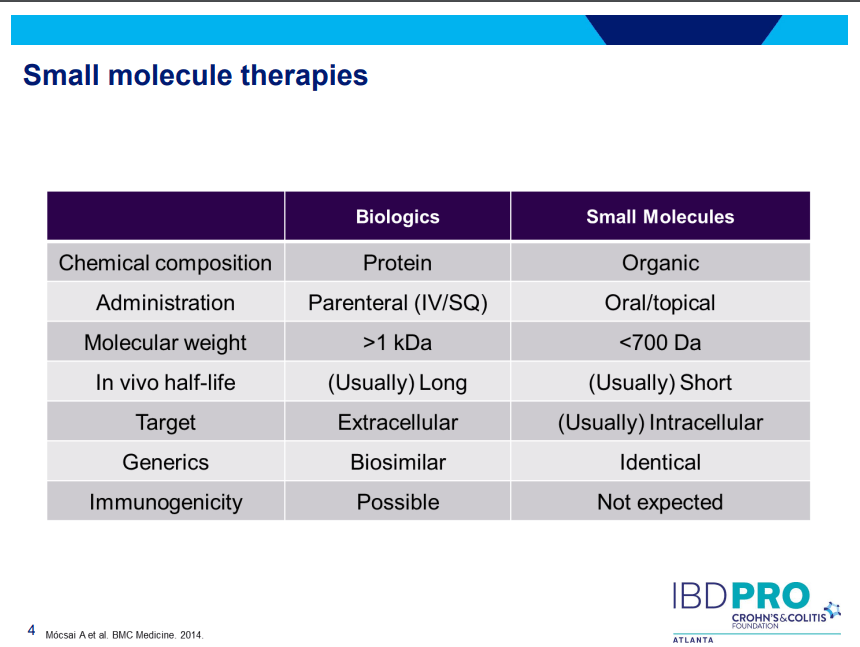
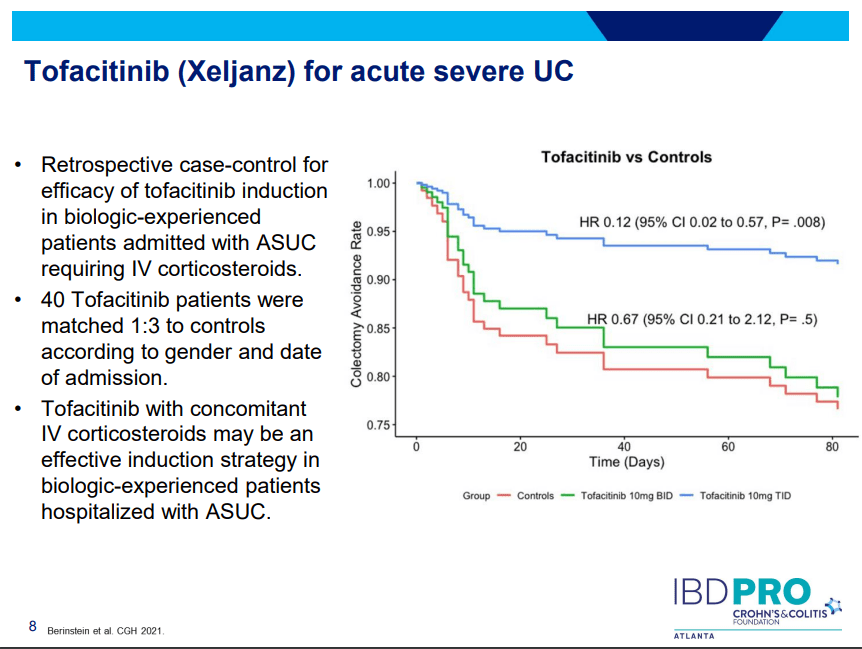
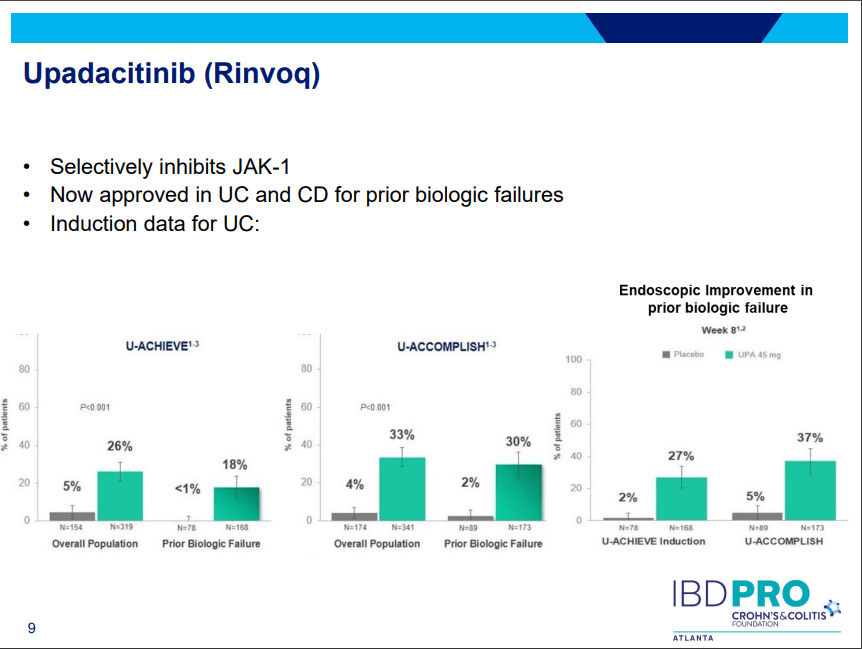
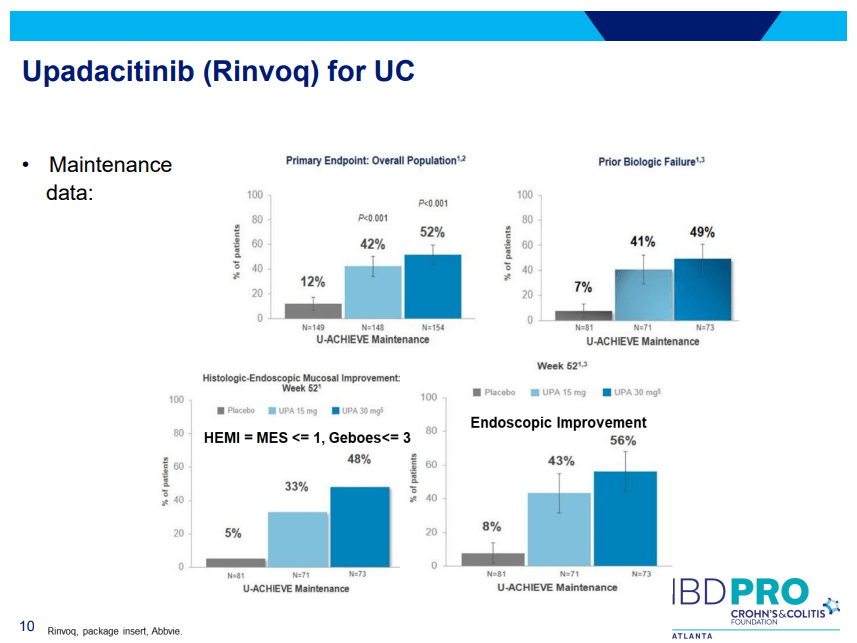
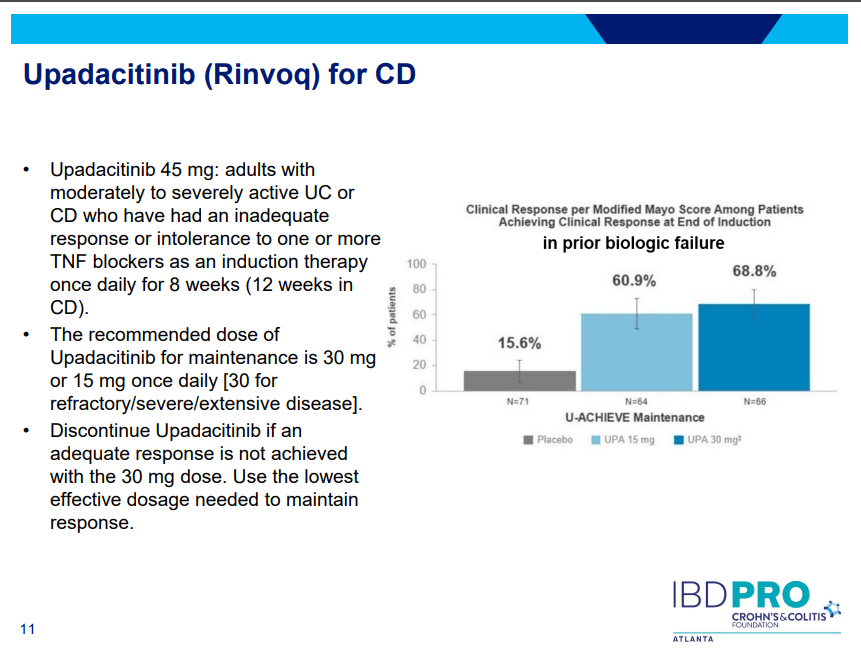
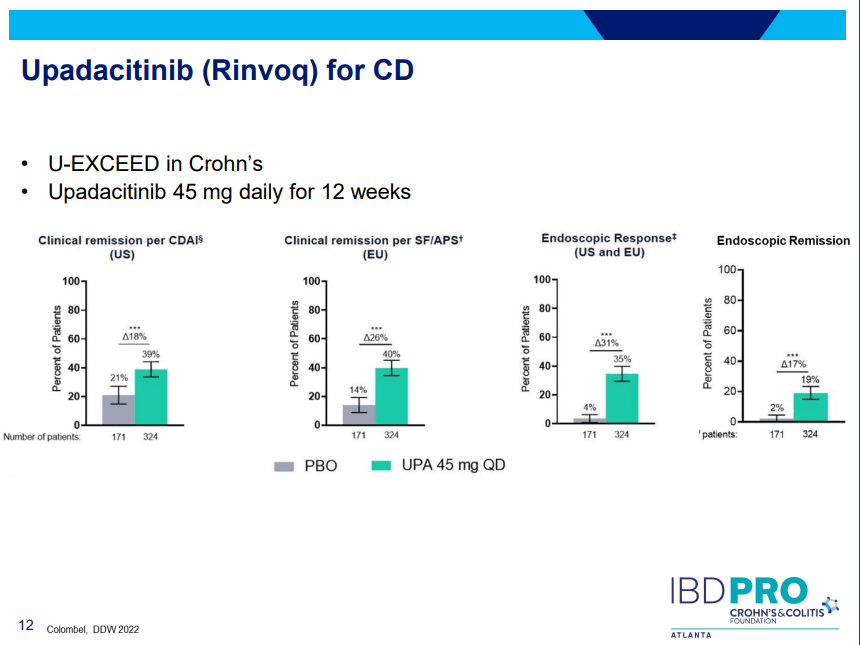
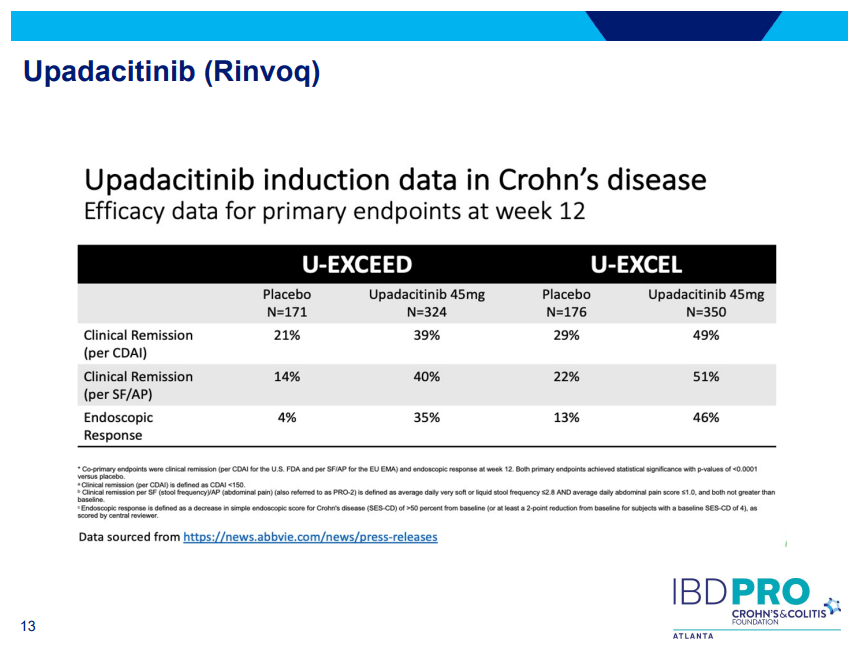
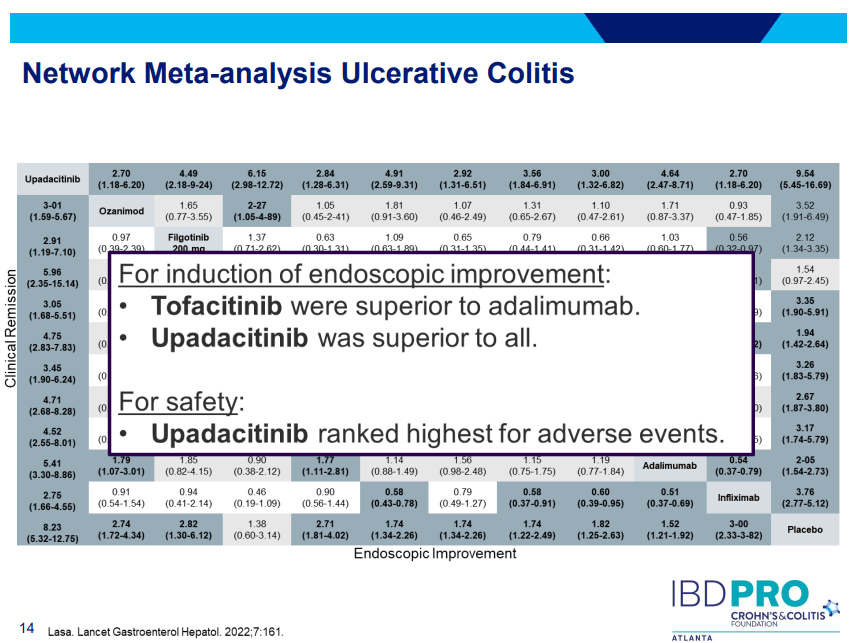
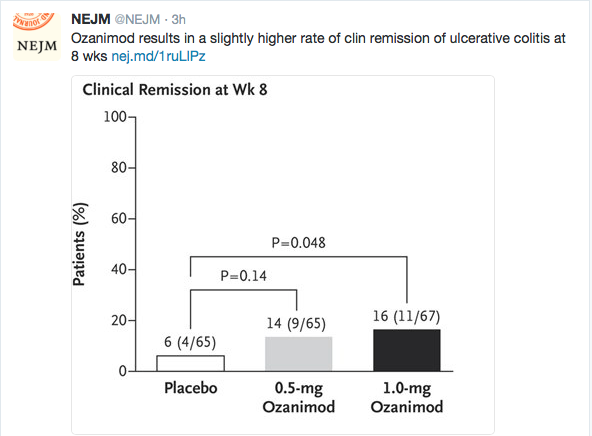
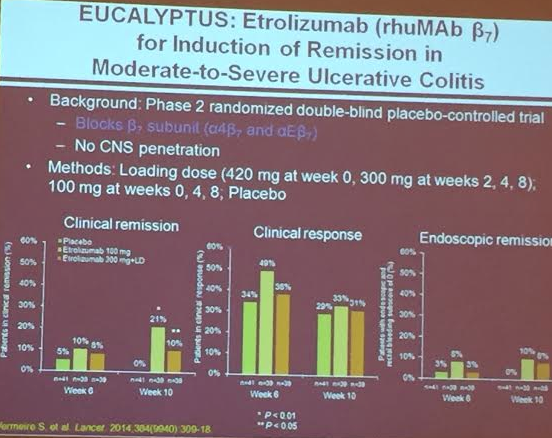
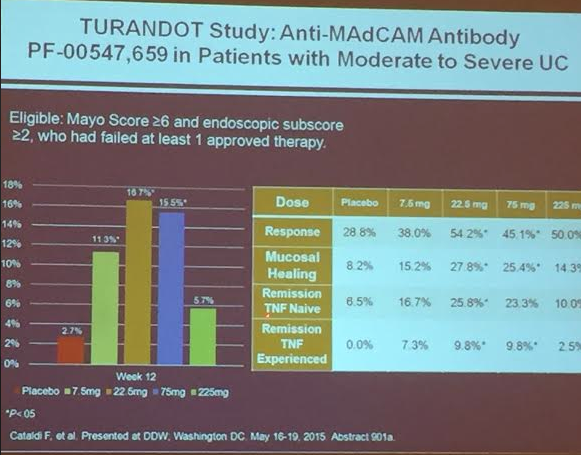
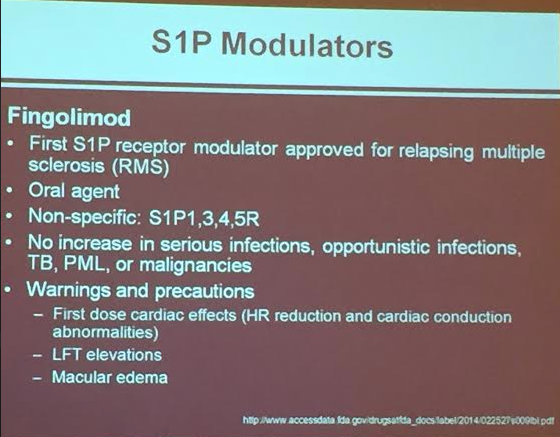
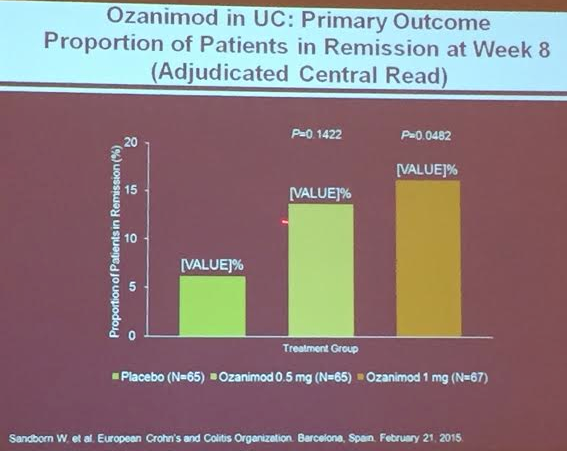
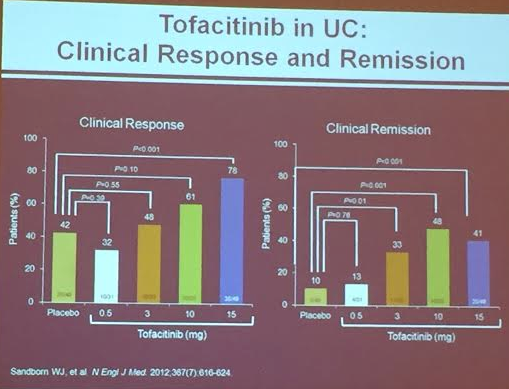
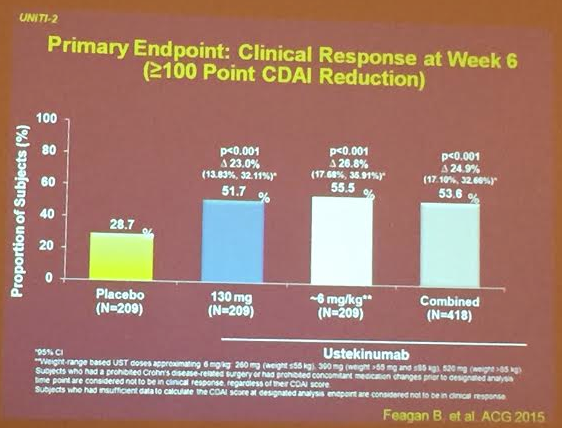
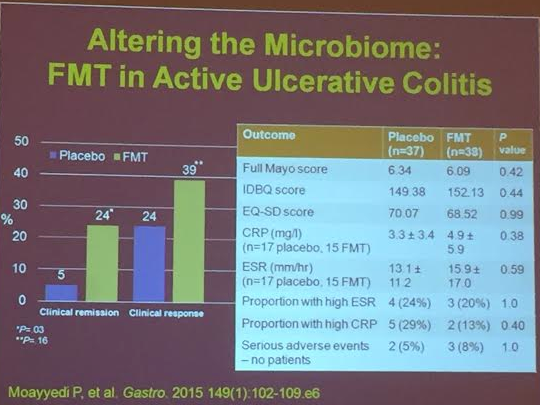
PS Dulai et al. Gastroenterol 2024; 166: 396-408. Open Access! Integrating Evidence to Guide Use of Biologics and Small Molecules for Inflammatory Bowel Diseases
“In this review, we provide a framework for clinicians and researchers to understand key differences in sources of evidence, how different methodologies are applied to study the comparative effectiveness of advanced medical therapies in IBD, and considerations for how these sources of evidence can be used to better integrate current guideline recommendations.”
This article explains the use of randomized controlled trials, “real-world evidence”/observational comparative studies, network meta-analysis, and post-hoc comparisons from randomized studies.
“The authors advocate for “”Given the rapidity with which new advanced medical therapies are becoming available in IBD, which quickly make current guidelines obsolete, living guidelines may offer a unique consideration to ensure applicability to routine care.”
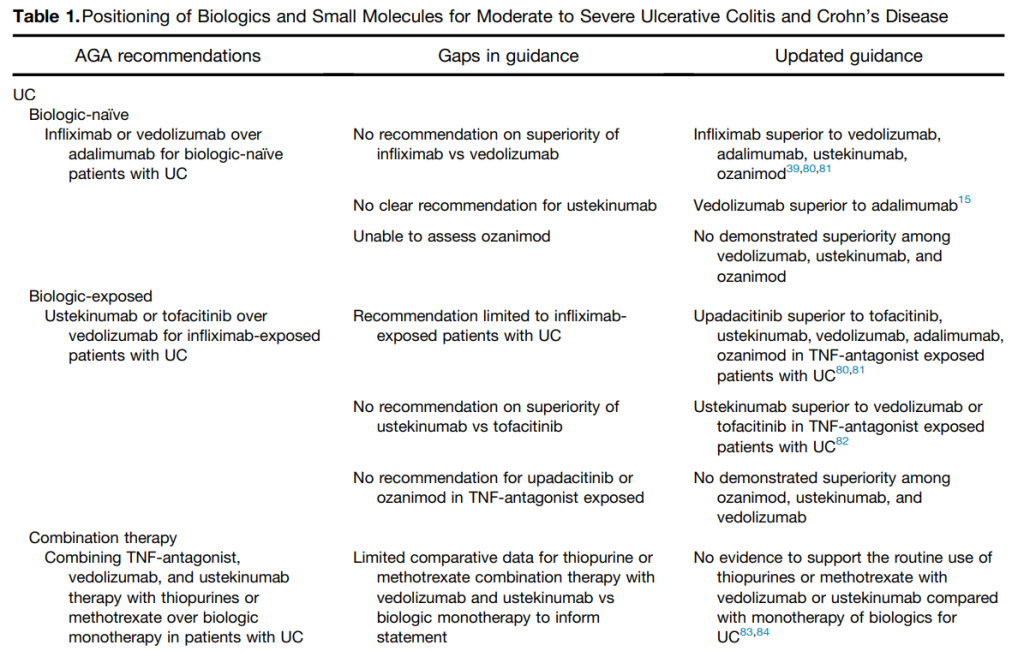

My take: This article provides a useful update of current advanced therapies and information in positioning these advanced therapies. It would be a great service if the IBD community could create something similar to HCVguidelines.org. The latter was a coordinated effort by the AASLD and IDSA to help provide expert advice during a deluge of amazing advances in HCV. And just like HCVguidelines, it is important to address “special” populations including pediatric patients and patients with very early onset IBD.
Related blog posts:
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis (2024)
- Impressive Results for Risankizumab in Refractory Crohn’s Disease (2024)
- Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial, Vedolizumab More Effective Than Adalimumab for Ulcerative Colitis
- IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 4
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.